When, after akds, you can do the next vaccination. Akds- "vaccine": composition and methods of safety studies. Decoding and composition
Issue 8. DTP.
What is DTP?
DTP (international abbreviation - DTP) is a combination vaccines against diphtheria, tetanus and pertussis. DTP vaccines are among the most reactogenic (i.e., capable of provoking adverse reactions) drugs. This is due to both the high content of antigens and their properties - the most reactogenic components of DTP vaccines are pertussis and, to a lesser extent, diphtheria.
The composition of the vaccine, reactions to administration (declared by the manufacturer)
1. TETRAKOK
3. INFANRIX
1. TETRAKOK
ADSORBED VACCINE FOR PREVENTION OF DIPHTHERIA, WHOLE, TETANUS AND POLIOMYELITIS
COMPOSITION AND APPEARANCE
One dose of the vaccine (0.5 ml) contains:
Active Ingredients:
Purified diphtheria toxoid> 30 IU
Purified tetanus toxoid> 60 IU
Inactivated Bordetella pertussis> 4 ME
Inactivated poliomyelitis virus type 1 40 D - antigenic units
Inactivated poliomyelitis virus type 2 8 D - antigenic units
Inactivated poliomyelitis virus type 3 32 D - antigenic units
Other Ingredients:
Aluminum hydroxide< 1, 25 мг
Formaldehyde< 0, 1 мг
Phenoxyethanol< 5 мкл
Twin 80
HANKS 199 medium containing: amino acids, mineral salts, vitamins and other substances dissolved in water for injection, the pH of which is adjusted between 6, 8 and 7, 5 using of hydrochloric acid or sodium hydroxide
The vaccine is a cloudy, whitish suspension.
INDICATIONS
Combined prophylaxis of diphtheria, pertussis, tetanus and poliomyelitis.
The drug is indicated for:
* primary vaccination of children from 2 months of age;
* revaccination of children from the second year of life.
According to the National calendar preventive vaccinations Russian Federation, primary vaccination against diphtheria, tetanus, pertussis and poliomyelitis is carried out from 3 months of age, and revaccination - at the age of 18 months.
The vaccine can be used up to 6 years old inclusive.
CONTRAINDICATIONS
The drug MUST NOT be used in following cases:
* Progressive encephalopathy with or without seizures (neurological pathology).
* A strong reaction that developed within 48 hours after the previous vaccination: an increase in body temperature up to 40 C and above, prolonged unusual crying syndrome, febrile or afebrile convulsions, hypotonic - hyporeactive syndrome.
* Hypersensitivity after previous vaccines to prevent diphtheria, tetanus, pertussis and polio.
* Known allergy to any ingredient in the vaccine (anaphylactic shock, Quincke's edema, generalized rash).
WARNINGS
Use with CAUTION if:
* Hypersensitivity to neomycin, streptomycin, polymyxin B and formaldehyde in connection with the use of these substances in the manufacture of the drug.
* Elevated body temperature, as well as acute or chronic illness in the acute stage (in this case, it is recommended to postpone vaccination until recovery or remission).
ADVERSE REACTIONS
As with any vaccine product, this vaccine can cause adverse reactions of varying degrees of severity in some patients.
* Soreness, redness, induration or swelling at the injection site may develop within 48 hours after vaccination and persist for several days. These reactions can be accompanied by the formation of a subcutaneous nodule that persists for several weeks. In very rare cases, sterile abscesses may occur.
* An increase in body temperature above 38, 5 C (on average, in 4% of vaccinated people), as well as prolonged unusual crying can be observed in the first 24-48 hours after injection.
* Allergic reactions: rash, urticaria, and, in very rare cases, anaphylactic shock or Quincke's edema (similar to urticaria with rapidly developing swelling of the face and neck) are possible.
* In rare cases, hypotonic-hyporeactive syndrome, prolonged crying syndrome, febrile or afebrile seizures may occur.
* In extremely rare cases, acute encephalopathy is noted. Neurological disorders due to vaccination, they are most often caused by the pertussis component of the vaccine.
2. Adsorbed diphtheria-tetanus toxoid liquid (ADS-toxoid)
ADS-toxoid consists of a mixture of purified diphtheria and tetanus
toxoids adsorbed on aluminum hydroxide.
One inoculation dose (0.5 ml) contains 30 flocculation units (Lf)
diphtheria toxoid, 10 binding units (EC) of tetanus toxoid, not more than 60 μg of merthiolate (preservative) and not more than 0.55 mg of aluminum hydroxide (sorbent).
Suspension of yellowish-white color, separating, when lagging behind, into a transparent supernatant liquid and a loose precipitate, which completely breaks down with shaking.
Immunobiological properties. Administration of the drug in accordance with
the approved scheme causes the formation of a specific antitoxic
immunity against diphtheria and tetanus.
Appointment. Prevention of diphtheria and tetanus in children under 6 years of age
age.
ADS-toxoid is used:
1. Children who have had whooping cough (from 3 months of age to 6
years of age).
2. Children with contraindications to the administration of the DPT vaccine.
3. Children aged 4-5 years inclusive, not previously vaccinated against diphtheria
and tetanus.
The course of vaccination consists of two vaccinations with an interval of 30 days. Reduction
no interval allowed. If it is necessary to increase the interval, another
vaccination should be carried out as soon as possible, determined by the condition
health of the child. Revaccination with ADS-toxoid is carried out once after 6-12
months after the completed course of vaccination. The first revaccination of children who have reached 6
years, as well as subsequent age-related revaccinations are carried out with ADS-M-toxoid.
ADS-toxoid can be administered a month later or simultaneously with polio
vaccine and other drugs of the national calendar of preventive
vaccinations.
Reaction to the introduction. ADS-toxoid is a weakly reactive drug. Individual vaccinated in the first two days may develop short-term general (fever, malaise) and local (soreness, hyperemia, swelling) reactions. In extremely rare cases, allergic reactions may develop (Quincke's edema, urticaria, polymorphic rash), a slight exacerbation allergic diseases... Considering the possibility of development allergic reactions immediate type in especially sensitive persons, the vaccinated must be monitored for 30 minutes. Vaccination sites should be provided with anti-shock therapy.
Contraindications Permanent contraindications to the use of ADS-
toxoid in adults and children are absent.
Children who have had acute illnesses are vaccinated 2-4 weeks after
recovery. In mild forms of diseases (rhinitis, slight hyperemia of the pharynx, etc.), vaccination is allowed after the disappearance of clinical symptoms.
Sick chronic diseases vaccinated upon reaching full or partial remission. Children with neurological changes are vaccinated after excluding the progression of the process. Patients with allergic diseases are vaccinated after 2-4 weeks of remission, while stable manifestations of the disease (localized skin phenomena, latent bronchospasm, etc.) are not contraindications to vaccination, which can be carried out against the background of appropriate therapy.
3. INFANRIX
DIPHTHERIA-TETANUS VACCINE, THREE-COMPONENT CELLLESS WHOLE ADSORBED LIQUID
COMPOSITION
The vaccine contains diphtheria toxoid, tetanus toxoid, and three purified pertussis antigens (pertussis toxoid (CA), filamentous hemagglutinin (PHA) and outer membrane protein (pertactin) with a molecular weight of 69 kDa, adsorbed on aluminum).
One dose of the vaccine (0.5 ml) contains:
Active ingredients: at least 30 international units (IU) of diphtheria toxoid, at least 40 IU of tetanus toxoid, 25 μg CA, 25 μg PHA and 8 μg pertactin.
Other ingredients: aluminum (in the form of aluminum hydroxide) - 0.5 mg, 2-phenoxyethanol (preservative) - 2.5 mg, sodium chloride - 4.5 mg, water for injection - up to 0.5 ml.
Residual formaldehyde content - no more than 0.2 mg / ml.
PURPOSE
Primary vaccination against diphtheria, tetanus and pertussis in children from 3 months of age.
Revaccination of children who were previously immunized with three doses of acellular pertussis-diphtheria-tetanus or whole-cell pertussis-diphtheria-tetanus vaccine.
At the beginning of the course of vaccination with whole-cell pertussis-diphtheria-tetanus vaccine, it is possible to administer subsequent doses of acellular pertussis-diphtheria-tetanus vaccine and vice versa.
CONTRAINDICATIONS
Known hypersensitivity to any component of this vaccine, as well as if the patient has experienced symptoms of hypersensitivity after the previous administration of Infanrix®.
Severe reaction (temperature above 40C, hyperemia or edema more than 8 cm in diameter) or complication (collapse or shock-like state that developed within 48 hours after vaccine administration; continuous crying lasting 3 hours or more that occurred within 48 hours after vaccine administration ; convulsions, accompanied or not accompanied by a febrile state, occurred within 3 days after vaccination) for the previous administration of Infanrix® vaccine.
Encephalopathy that develops within 7 days after the previous administration of the vaccine containing the pertussis component. In this case, the course of vaccination should be continued with diphtheria-tetanus vaccine.
ADVERSE REACTIONS
Pain, redness (more than 2 cm), swelling (more than 2 cm), fever,
unusual crying, vomiting, diarrhea, refusal to eat or drink, drowsiness, restlessness.
Cases of collapses and shock-like states (hypotonic-hyporesponsive episodes) and convulsions within 2-3 days after vaccination were extremely rare. These side effects were transient and did not lead to any consequences.
Also, during the clinical use of the vaccine, the following adverse events were recorded in the post-vaccination period.
On the part of the skin (1% or less): dermatitis;
from the side respiratory system(3% or less): cough, rhinitis, bronchitis, other upper respiratory tract infections;
associated with resistance to infections (1% or less): otitis media.
After revaccination with Infanrix® vaccine, carried out after primary vaccination with the same vaccine (according to studies covering 2,363 injected doses)
Respiratory system disorders (4% or less): cough, pharyngitis, bronchitis, other upper respiratory tract infections, rhinitis, respiratory failure;
associated with resistance to infections (3% or less): viral infection, otitis media.
After revaccination with Infanrix® vaccine, carried out after primary vaccination with whole-cell pertussis-diphtheria-tetanus vaccine (according to studies covering 606 doses administered)
From the respiratory system (3% or less): cough, pharyngitis, upper respiratory tract infections, bronchitis;
associated with resistance to infections (2% or less): otitis media.
SPECIAL INSTRUCTIONS
Before vaccination, the child's history should be studied, paying attention to the previous administration of vaccines and the associated occurrence of adverse reactions, as well as an examination.
The introduction of the vaccine should be postponed if the child has an acute illness accompanied by an increase in temperature. In case of an infectious disease in a mild form, vaccination can be carried out after the temperature has returned to normal. As with the introduction of any other vaccines, you should have everything you need ready to stop a possible anaphylactic reaction to Infanrix®. Therefore, the vaccinated must be kept under medical supervision within 30 minutes after immunization.
Infanrix® should be used with caution in patients with thrombocytopenia or with disorders of the blood coagulation system, since in such patients intramuscular injection may cause bleeding. To prevent bleeding, press on the injection site without rubbing it for at least 2 minutes.
HIV infection is not a contraindication to vaccination.
Contraindications to the introduction of DPT
MODERN CONTRAINDICATIONS TO DTP
1. Sharp infectious diseases and non-infectious - vaccination is carried out no earlier than 1 month after recovery;
2. Exacerbation of chronic diseases - vaccination is carried out individually in 1-3 months from the onset of remission;
3. Long-term and serious illnesses(viral hepatitis, tuberculosis, meningitis, myocarditis, hemorrhagic vasculitis, etc.) - vaccination is carried out individually 5-12 months after recovery;
4. Unusual reactions and complications to the previous administration of DTP vaccine
- severe forms allergic reactions (shock, Quincke's edema, polymorphic exudative erythema);
- convulsions, episodes of a shrill scream, disturbance of consciousness;
- severe extensive reactions (temperature rise over 39.5C, severe symptoms of intoxication);
5. Diseases of the nervous system, convulsive syndrome, general or local neurological symptoms;
6. Prematurity (birth weight less than 2500 g) - vaccination is carried out at the age of 6 months, subject to normal psychomotor and physical development;
7. Severe forms of allergic diseases: shock, serum sickness syndrome, recurrent Quincke's edema, generalized eczema, severe forms bronchial asthma;
8. Immunodeficiency states, malignant blood diseases and neoplasms, the appointment of immunosuppressants.
CONTRAINDICATIONS TO DTP THAT EXISTED 30 YEARS BEFORE THE INTRODUCTION OF "NEW" ...
Clinical contraindications for vaccinations
All healthy children are subject to vaccinations, who must be preliminarily examined by a doctor (paramedic at the paramedic-obstetric station), taking into account their anamnesis data (previous diseases, reactions to previously vaccinated, allergic reactions to medications, food products, etc.) and examined with compulsory thermometry immediately before vaccination. If necessary, urine and blood tests are done. Children with chronic diseases and allergic conditions living in rural areas must be examined by a doctor before the vaccination. Parents of children attending nursery preschool institutions must be notified in advance of the date of vaccination.
When selecting children for DTP vaccination, contraindications must be strictly observed; it should be borne in mind that some children who have contraindications to the administration of the DPT vaccine can be vaccinated against diphtheria and tetanus with ADS toxoids.
Below is a differentiated list of contraindications for immunization with DTP vaccine and DTP toxoid.
Medical contraindications for inoculation with DTP-vaccine, DTP and DTP-M toxoids
1. Acute diseases(infectious and non-infectious), including the period of convalescence: Not earlier than a month after recovery
* Infectious hepatitis (hepatitis A): Not earlier than 6 months after recovery
* Serum hepatitis (hepatitis B): Not earlier than 12 months after recovery
* Meningococcal infection (generalized form without meningitis), infectious diseases with a protracted and chronic course (sepsis, dysentery, otitis media, etc.): Not earlier than 6 months after recovery
* Chronic tonsillitis and adenoiditis requiring surgical treatment: Not earlier than 2 months after surgery or debridement according to the otolaryngologist's opinion
2. Tuberculosis: pulmonary and extrapulmonary forms in the open phase; severe tuberculous intoxication with subfebrile condition; turn of tuberculin tests: After recovery according to the conclusion of the phthisiatrician
3. Chronic pneumonia:
ADS-toxin - Not earlier than 12 months from the date of remission
ADS-M toxoid - Not earlier than 6 months from the date of remission
4. Allergic diseases
Anaphylactic shock, history of serum sickness, recurrent Quincke's edema, widespread urticaria, Lyell and Stevens-Jones syndromes: Contraindicated
* Bronchial asthma, asthmatic bronchitis:
DPT vaccine - Contraindicated
ADS-M toxoid - Not earlier than 2 years from the onset of remission (according to the opinion of the allergist)
* Common eczema, neurodermatitis, scrofulus:
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - Not earlier than 12 months from the onset of remission
* Allergic reactions to certain allergens (various rashes and other clinical disorders): Not earlier than 3 months after the reaction
* History of DPT vaccine reaction
a. an increase in temperature to 40C and above in the first two days;
b. severe allergic reactions
v. neurological complications (convulsive syndrome, piercing continuous cry on the first day)
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - Not earlier than 12 months after the reaction (according to the conclusion of a specialist)
5. Diseases of the nervous system:
Hereditary, degenerative and progressive diseases of the nervous system:
Contraindicated
* Epilepsy, history of convulsive syndrome:
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - Not earlier than 6 months after a seizure
* Birth injury with residual effects (children cerebral paralysis and etc.):
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - With favorable current forms over the age of one year
* Birth trauma, prolonged birth asphyxia without residual manifestations from the nervous system:
DPT vaccine - After one year of age
ADS-toxin - After the age of one year
ADS-M toxoid - After six months
* Hydrocephalus de- and subcompensated: Contraindicated
* Compensated hydrocephalus:
DPT vaccine - With stable compensation throughout the year
ADS-toxin - With stable compensation for at least 6 months
ADS-M toxoid - With persistent compensation for at least 6 months
* Children from the group high risk(the threat of a miscarriage in the mother, obstetric benefits or surgical interventions during childbirth):
DTP vaccine - After 6 months of age
ADS-toxin - After 3 months of age
ADS-M toxoid - After 3 months of age
* Infectious diseases of the central nervous system (meningitis, encephalitis, encephalomyelitis) with residual effects:
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
* Without residual effects:
ADS-M toxoid - Not earlier than 6 months after the end acute period
* Traumatic brain injury (concussion, bruises, hemorrhages in the brain and membranes) with residual effects:
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - Not earlier than 2 years after the end of the acute period
* Without residual effects:
DTP vaccine - 12 months after the end of the acute period
ADS-toxin - 12 months after the end of the acute period
ADS-M toxoid - Not earlier than 6 months after the end of the acute period
6. Severe forms of rickets (II-III), malnutrition (II-III), vitamin deficiency: After recovery
7. Hemolytic disease of the newborn. Severe prematurity (Weight less than 2 kg.): After the age of 1 year, with normal indices overall development and blood
8. Diseases of the cardiovascular system:
decompensated congenital and acquired heart defects; subacute septic endocarditis: Contraindicated
* Heart defects in a state of compensation: According to a specialist
* Rheumatism:
DPT vaccine - Contraindicated
ADS-toxin - Not earlier than 3 years from the moment of clinical and laboratory remission
ADS-M toxoid - Not earlier than 3 years from the moment of clinical and laboratory remission
* Myocarditis:
DPT vaccine - Contraindicated
ADS-toxin - Not earlier than 12 months from recovery according to the conclusion of a specialist
ADS-M anatoxin - Not earlier than 12 months from recovery according to the conclusion of a specialist
9. Kidney disease
Chronic renal failure, congenital nephropathy: Contraindicated
* Diffuse glomerulonephritis:
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - 5 years after complete clinical and laboratory remission
* Pyelonephritis:
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - 3 years after complete clinical and laboratory remission
* Toxic nephropathy (transient): Not earlier than 6 months after recovery
* Infections urinary tract:
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - 12 months after complete clinical and laboratory remission
10. Diseases of the liver and pancreas
Liver cirrhosis, chronic hepatitis and pancreatitis: Contraindicated
* Acute pancreatitis:
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - Not earlier than 6 months after recovery
* Inflammatory diseases biliary tract:
DPT vaccine - Not earlier than 6 months after recovery (subject to bile readjustment)
ADS-toxin - Not earlier than 6 months after recovery (subject to bile readjustment)
ADS-M toxoid - Not earlier than 3 months after recovery
11. Diseases of the blood
leukemia, lymphogranulomatosis, aplastic anemia, hemophilia, Verlhof's disease, constitutional dysgammastobulinemia: Contraindicated
* Hemorrhagic vasculitis (capillarotoxicosis):
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - Not earlier than 2 years from the date of complete clinical and laboratory remission
* Deficiency anemias: After recovery
12. Malignant neoplasms: Contraindicated
13. Collagenosis: Contraindicated.
14. Diseases of the endocrine system
diabetes mellitus, severe forms of thyrotoxicosis, adrenal insufficiency (or dysfunction), myxedema, congenital fermentopathies: Contraindicated
* Thymomegaly:
DPT vaccine - Contraindicated
ADS-toxin - Contraindicated
ADS-M toxoid - Upon the onset of age-related involution
15. Ulcerative colitis: Contraindicated.
16. Operative interventions: Not earlier than 2 months after surgery
DTP - "vaccine": composition and methods of studying safety
“I would like to note another annoying circumstance that exists among some specialists in justification of adverse vaccination reactions. It is believed that with sufficient effectiveness of vaccines, their reactogenicity can be neglected. This kind of reasoning serves only as a disguise bad job vaccine authors and must meet strong objections. Preventive vaccines used for children should be completely harmless, minimally reactogenic and highly effective. Vaccination preparations of this characteristic, if desired and persistent, can be obtained. "L. F. Zdrodovsky
What is the purpose of adding mercury-containing salt - an antibacterial pesticide - to some inactivated vaccines?
A. I. Kondrusev (former chief state sanitary doctor of the USSR): "The merthiolate used in the production of DPT vaccine plays the role of a stabilizer of immunogenic properties (but no one has studied them! - G. Ch.) And suppresses the reactogenicity of the pertussis component" (together with formaldehyde ... suppresses reactogenicity? - G.Ch.) - "Komsomolskaya Pravda", 09.10.1988.
With such an answer, the question naturally arises: why is merthiolate introduced into ADS-M, ADS, AD, which do not contain "K" - the pertussis component? Or: why is merthiolate not used at all enterprises that manufacture serum preparations - immunoglobulins? Some manufactures, even domestic ones, do not use preservatives, but they produce sterile serum preparations.
T. A. Bektimirov ( former director GNIISK):
"1. The materials available at the institute and the WHO requirements for medical biological products do not give grounds for the immediate exclusion of merthiolate from the DPT vaccine and other sorbed drugs.
2. Considering that, according to the regulatory and technical documentation for immunoglobulins, the addition of merthiolate is not mandatory, a complete transition to the production of immunoglobulins without merthiolate is possible, while simultaneously improving the production conditions at enterprises producing preparations with merthiolate.
4 .... To develop production technologies to eliminate preservatives from biological products.
5. When new preparations containing merthiolate are received by the KVS or GISK, ask the authors to provide justification for the inclusion of a preservative in a biological product. "
Therefore, merthiolate cannot be excluded from sorbed preparations. Why? For additional clarification from other authors, see below. And another thing: it is not very clear whether to include preservatives in biological products or ... to develop new technologies?
A. A. Sumarokov (former chairman of the FAC): "... As well as the WHO recommendations, according to which not only it is allowed, but in relation to individual drugs, the use of merthiolate is strongly recommended, which guarantees the sterility of drugs both during their release and in application process ". - From the minutes of the PIC meeting, 02/04/87
And now let's turn to some of the recommendations of the WHO, which allegedly "strongly recommends the use of merthiolate": As a rule, proprietary names are distinguished by initial capital letters... ". I have not met a single mention of the WHO on the patenting of merthiolate, and there are no" strong recommendations "for its use (WHO, series of technical reports - STD, 1960-1990).
Merthiolate is a mercury-containing salt, it would seem that everything is clear. But we would never have known what kind of merthiolate is added to children's vaccines and purchased (or purchased for 40 years in advance) by our state ... for foreign currency, if it were not for the oversight of Yu. Ya. Yakushevich, director of the I. I. Mechnikov of Moscow (Petrovo-Dalnee). He asked GNIISK for clarification on the use of merthiolate (letters dated 22.06.84 and 04.12.84): "At present, the drug entering the USSR from Germany and Switzerland has an inscription -" only for laboratory purposes ", and from the USA (the company "Sigma") additionally - "do not use for drugs." The drug meets the requirements of Order No. 31, but these inscriptions raise doubts. "
These inscriptions, tragic for our children, cause only doubts among the manufacturer of vaccines used for babies. Not only that, for the last five years, the production workers and controllers of the State Research Institute of the State Scientific Inspection, as well as some health officials, have been trying to prove (without documents and experimental research) that the dose is ... small. For whom is it "negligible" and safe if there are no documents from companies, and GNIISK did not study it for safety? There is no data on the complex effect of merthiolate and formaldehyde, no one has ever studied this conglomerate - DPT with all chemical impurities - on long-term consequences... Firms warn, therefore, do not accept any responsibility. We received a reply from Soyuzkhimexport (25.12.86): environment, the production of merthiolate in Europe is prohibited, so the association failed to place your order for this drug (the firms did not accept our order). "
"So what," FF told me. Rezepov, - "and we will buy it in Africa, now new factories are being built there ... they have been producing such a DTP for fifty years and we will prepare another 100 in the same way ...". The same Rezepov, who is the main controlling head of the DPT.
DPT contains "acceptable, low" concentrations of two known for their effects pesticide disinfectants. It would seem so natural, before admitting, it is necessary to study and prove the safety of the admitted "small" doses. But they didn’t study it. Why did it happen? "This is what the WHO recommends", - the answer of the controllers of the State Research Institute of the Information Technologies and Communications of the Russian Federation. Well, let's turn again to the WHO recommendations:
- "... The mention of certain companies or products ... does not mean that WHO favors them ..."
- ".. If chemicals are used in the production process, they must be approved by the national control body ..."
- "... At the end of the process, any inactivating agent must be removed or neutralized ... The method used for this must be approved by the national control authority ..."
- "... It is necessary to choose such an inactivation technique so that the finished material does not contain detectable quantities chemical substances... "(the inactivating agent in DPT is formaldehyde, moreover, in quite detectable quantities - G.Ch.);
- "... It is especially important to make sure that finished product did not contain substances known to be toxic or cause allergic reactions in humans ... "
- "... Add only those preservatives and other substances which are approved by the national control authority and which, in the concentration applied, should be shown by the results of the relevant tests, do not impair the safety or efficacy of the vaccines ..."
- "... The term" national control authority "or" national control laboratory ", as used in the text of the WHO recommendations, always refers to the facilities in the country where the vaccine is produced."
So, it seems like there is no reason to refer to the "urgent recommendations" of this organization. Our health authorities are responsible for national orders, regulations, requirements and vaccine deficiencies.
In our talk, we often use the word "known". But what we talk about and write about is actually known for a long time. An immense amount of literature has been written about the toxic and allergenic properties of mercury salts and formaldehyde, including in domestic publications. The volume of materials is such that it is unrealistic to present it briefly. Information is given in reviews, reference books, and WHO publications.
In the "Handbook on pesticides" edited by Acad. L. I. Medveda said: "... Pesticides are a collective name generally accepted in world practice chemicals... pestle - harm, cido - I kill ... They have biological activity and can cause disruptions in the life of not only living organisms against which they are used, but also others, including animals and humans ... Possessing biological activity, they potentially hazardous to wildlife and human health ... Concentrations capable of destroying bacteria can be hazardous to human health. "Read - even more so for health infants with the injection of a complex of chemicals in doses that have not been studied for safety in any biological model.
It is known from other sources that:
The greatest tropism of mercury salts is towards the tissues of the fetus (embryo), which resulted in the birth of a large number children with congenital deformities, with lesions of the central nervous system, including cerebral palsy, chorea, ataxia, convulsions, mental retardation, rare forms of skeletal deformities - all this is caused by the consumption of mercury-containing fish in the diet of pregnant women. This is through the placenta, from ... fish, and we are injections to an infant;
Another flash, with a large number cases of poisoning and deaths was caused in Iraq by the consumption of contaminated bread baked from grain treated with alkyl mercury fungicides;
Merthiolate - sodium ethylmercury salicylate - analogue of granosan - seed dressing;
There is even evidence that the USSR is not allowed to use preservatives for consumer products (milk, butter, bread, fresh meat) ... especially in food baby food... For the physiological intake of food - preservatives are not allowed, but for injections for infants - please!
From the "Encyclopedia of Adverse Reactions":
"... Alkyl mercury compounds, methyl and ethyl, are not used in medicine ... These are highly toxic compounds ... They, unlike most other mercury compounds, are lipophilic, are slowly excreted from the body and therefore can accumulate in the nervous tissue ... ". It is this kind of merthiolate - ethylmercursalicylic acid of sodium salt - for more than three decades, regularly and "necessarily" (!) Introduced into the body of infants, and even after vaccination with weakened mycobacterium tuberculosis - BCG.
(G.P. Chervonskaya "Vaccinations: Myths and Reality")
Vaccination statistics
Deadly vaccination
Christina Kolebek
Today is such a sweet sixteenth birthday for my daughter, but we won't be celebrating it. Instead, I will light a candle in her memory, and when I blow it out, I will make a wish. My wish is for mothers all over the world to learn and be able to make decisions based on knowledge. This solution will prevent senseless tragedies and never allow mothers to experience my pain.
Laura's story
After forty-one weeks of pregnancy, healthy, adorable baby Laura Mary entered this world on July 27, 1986. At home we were enthusiastically greeted by our family and our friends, anxiously awaiting the arrival of a new family member. They filled her with so many beautiful little ones pink dresses that we were joking that she couldn't wear them out in her entire life.
Our life has changed completely. They now revolved around strolling in the park, visiting friends, changing diapers, feeding at night, and shopping for even smaller pink dresses. We became parents, we had now real family and life was wonderful.
I took Laura to the pediatrician for a checkup. The latter was a middle-aged, amiable and affable woman. She was very happy with Laura's development and weight at 3 months. She got her DPT and oral polio vaccines. I didn't ask any questions, because I knew that all my friends' children received the same vaccinations at the same age and that all "good mothers" vaccinate their children to protect them. We left the pediatrician's office and went home.
Laura was very restless. It was unusual for her. She was crying loudly in her wheelchair. When we got home, I realized she was urinating so much that everything in the stroller got wet. Then her crying turned into a scream, then she got a fever. The leg is swollen, red and hot to the touch. I called the pediatrician and she said that it was "normal" and that I should give the child tempra. I gave tempra and felt calmer, because the pediatrician convinced me that this is normal.
Laura continued to scream, and I could no longer calm her down. All my instincts told me that it was not normal, but I was young, this was my first child and I trusted the doctor. I could not hold Laura in my arms, because in my arms she began to scream even louder - it seemed that any movement of her leg caused her terrible pain. I put her in the crib and she screamed until she fell asleep. This gave me great relief. Tempra was working and the doctor was probably right. I thought it was foolish to worry so much. Later a short time Laura woke up screaming and spent the whole evening screaming and falling asleep.
She had absolutely no appetite, and nothing could stop her screaming. Finally it was bedtime and she screamed in her crib until she fell asleep. She had never screamed before before going to bed, and I felt very uncomfortable that I had left her, but when I held her in my arms, she screamed even louder. My husband came home from work and I told him everything that happened that day. Laura was fast asleep in her crib; she seemed to be feeling better and we decided not to worry ... And I should have worried!
In the morning I woke up and was amazed that my husband slept. I knew right away that something was wrong, and my anxiety last night enveloped me again. Terrified, I ran to her bed. Laura looked somehow unnatural. I closed my eyes and opened them again, thinking it was a dream. But when I opened my eyes, she looked dead.
I watched him do artificial respiration. I was numb and could not move. He tried to revive our child, but in vain. He shouted for me to open the door for the paramedics. For a while, I returned to reality, went and opened the door. Now I could move, but I could not speak. I stood in a daze, shaking my head and feeling completely helpless as the paramedics, police and firemen ran past me into our house. I didn’t cry, I wanted to shout to them to leave her alone, but I couldn’t speak. She was lying on the floor, and they shook her tiny body in a small bedroom with walls painted in yellow, and wallpaper with clowns. I stood there, praying in my soul that they would leave her alone, that they would come out of her bedroom and that I would wake up from this terrible dream.
I heard someone say there was a weak pulse, and hope arose in me. Ambulance rushed Laura to the hospital. At this time, the detectives ushered us into another room, and the interrogation began.
They decided that my husband and I should be interrogated in different rooms... I knew right away that they suspected us. We all know that healthy children do not die suddenly and just like that. I was speechless. I had already decided that it was all my fault, one way or another, and although I was not completely sure what exactly I did to kill her, I was convinced that somehow I was the cause of everything. Perhaps I was punished by God for my sin, or perhaps it was because I left her screaming until she fell asleep that night. The fact remained: my child was dead, and the children of "good mothers" did not die.
The husband began to strongly object to the nature of the questions asked and demanded that we be taken to the hospital immediately so that we could see our child. The detectives finally took us to the hospital. There we were taken to the "bad news room". The doctor came in and insisted that we sit down before he spoke. He began to talk about the fact that they had tried this and that, and finally, he said words that will echo in my ears all my life:
"She died".
The pediatrician, whom I respected and admired so much, broke down and cried when I told her this news by phone. She couldn't decide what to do. She defended the vaccine, then blamed her and those who said it was safe for my child's death.
Then she told me that she had another baby, a boy, who died after the same vaccination.
The detectives took us home. They asked so many questions and repeated them so often that they finally got tired of it themselves. Everything revolved around our possible involvement in the case. They searched everything for signs of illegal entry into the house. The husband told them again and again about unusual behavior child from the time he was vaccinated.
All our friends visited us. I made coffee and tidied up the house like it was an ordinary day we had guests. Shock is a strange and wonderful thing at the same time. You do not understand what is happening to you.
My parents insisted that I move in with them for a few days while my husband and friends were doing terrible job collecting things in the nursery. The room that I decorated with such love has become so empty and a source of such pain ...
A few days later, after a funeral and a tiny white coffin that was so small that my husband carried it alone, I finally came out of my shock and allowed myself to cry. I sobbed about everything that will never happen in my life. About a ballet studio to which I will never take my daughter. About her wedding, which I will never attend. About the grandchildren I will never see. About all the dreams associated with a daughter that will never come true. I sobbed about everything that happened and everything that will never happen. There was a void in me that threatened to swallow me whole. They were the blackest, the most scary days in my life.
The detectives eventually made sure that we did not harm our daughter in any way, and the investigation into the causes of her death was completed. We were left with no answers.
The doctors were reluctant to talk about her death in the context of any connection with the vaccine and, one by one, refused to answer our questions. I have been told repeatedly that "the benefits outweigh". I was even told that vaccine deaths were "expected" in the war against disease, but that these losses were considered to be at an "acceptable" level. However, for me, for a mother who lost her child, this did not seem acceptable.
Finally, a few months later, the coroner told us that the cause of death was SIDS (Sudden Infant Death Syndrome), which means "cause is unknown," and refused to give us a copy of the autopsy report.
It took almost a year for us to receive this protocol. To our dismay, we realized that the autopsy results were copied directly from the vaccine monograph, from the Contraindications section. The following was written:
“Cases of sudden infant death syndrome have been reported following vaccination with a vaccine containing diphtheria, pertussis and tetanus components. However, the significance of these reports is unclear. sudden infant deaths falls at the age of 2 to 4 months "
No toxicological testing was performed and the pediatrician never reported adverse reactions to the vaccine to health officials. I later learned that most vaccine-related deaths are attributed to SIDS, and SIDS statistics are NOT included in the vaccine adverse reaction data, even if the child dies a few hours after the vaccine. With this data, doctors and the public are convinced that vaccinations are safe.
The government's own literature reports that there has been little or no research on the safety or efficacy of vaccines. In essence, our children - this is it, this test. According to the literature, immunization is the “most cost effective” way to prevent disease. Nowhere in their literature is it stated that at the same time it is the safest. We are trading our children's lives to save government money. We are told the benefit outweighs the risk, but many of the diseases we vaccinate children against are not life-threatening when vaccines can kill.
Vaccinations kill more often than they try to convince us. We play vaccine roulette with the lives of our children, and we never know which child will fall victim to the next vaccine.
Are you willing to risk a 1 in 500,000 chance of death, 1 in 100,000 for permanent brain damage, 1 in 1,700 for seizures and convulsions, or 1 in 100 for adverse reactions? Are these odds reasonable enough to convince you to play roulette with your child's life?
I assure you that death by vaccination is neither quick nor painless. I watched helplessly as my daughter died slowly and painfully, screaming and writhing in pain as the vaccine did what it was designed to do — destroy her fragile immune system. Poisons used as preservatives seeped into her tiny body, penetrated into her organs and destroyed them until they completely stopped working. This picture will always haunt me, and I believe that neither parent should ever witness it.
Considered too inhumane for the county's most violent criminals, the death sentence was pronounced for my wonderful, innocent daughter, and executed by lethal injection.
Today, on my daughter's birthday, I mourn not only the loss of my own child but also all innocent children for whom the benefits of vaccines did not outweigh the risks, and who were sentenced to death by lethal injection under the slogan "the benefits outweighs." True war is not waged against disease; somehow we have become our own worst enemies by substituting our faith in science for nature. Today I call on all mothers in the world to join me and put an end to this senseless extermination of the most precious thing - our children.
---------------------
In the next issue: Poliomyelitis
Mailing host: Aglaya Yakovleva ( [email protected])
Health to you!
Doctors call the DPT vaccine the most important for childhood immunization, since it grants immunity in a complex manner to 3 dangerous infectious diseases: diphtheria, whooping cough and tetanus. Regardless of which vaccine a child or an adult will be vaccinated, vaccination is carried out according to the same scheme for each age. For children under one year old, these are three vaccinations with an interval of one and a half months, starting from 3 months of age, followed by revaccination in a year and a half. Older children (according to the schedule at 7 and 14 years old) and adults need to be vaccinated with another drug, without the pertussis component, or with one of the imported DTP. Let's take a closer look at all the available drugs in order to figure out which DPT vaccine is still better.
Domestic vaccine
On the territory of Russia, the drug is manufactured by NPK Microgen. The drug meets all the necessary requirements for the quality and effectiveness of immunization. In its composition, the drug contains three main components: toxoids of the causative agents of diphtheria and tetanus and cells of inactivated (dead) whooping cough. They act according to the principle standard for all vaccines - getting into the bloodstream, they cause a stress reaction of immunity, which develops to hazardous components antibodies. Subsequently, these antibodies prevent the spread of this infection in the body. Despite numerous controversies around the domestic DPT, it is effective and completely safe. However, the vaccine cannot be completely harmless to the body and the domestic drug is far from being in the first place here: it contains harmful substances formalin is also merthiolate. In addition, whole cells of the pertussis bacillus, which is an immunogenic component, have a high stress effect on the immune system and the body as a whole, as a result of which children or people with weakened immunity can get sick or undergo unpleasant post-vaccination reactions.
Do not hesitate to ask your doctor about which vaccine to choose in an unusual situation.
DPT from "NPK Microgen" is produced in glass medical ampoules, 5 or 10 pieces in a cardboard mesh packaging. Each ampoule contains 0.5 ml of the drug, which is one standard dose for inoculation. The kit always includes instructions and a special knife for opening ampoules. The main argument in favor of being vaccinated with a domestic vaccine is the economic benefit: in polyclinics it is provided free of charge, and in pharmacies it is sold for an amount not exceeding 200 rubles per pack (5-10 doses).
Imported vaccines
Vaccines produced in other countries for the prevention of diphtheria, tetanus and pertussis are conventionally also called DPT, although this is the direct name of the drug produced in Russia. It is worth talking about them in more detail, since each imported vaccine is significantly different from the Russian one. The main difference is the absence of formalin and merthiolate in the composition (these substances are prohibited for use in drugs on the territory of the European Union and the USA) and the cell-free technology for the production of the anti-pertussis component. Such features of the composition significantly reduce possible post-vaccination reactions, such as fever, swelling, rash, convulsions, and negate the chance of an allergic reaction. In addition, many imported DPT vaccines set up against others dangerous infections, poliomyelitis, hepatitis, etc. The immune response (vaccination efficiency) is 2-3% lower than that of the Russian vaccine, however, taking into account revaccination, there is practically no difference. 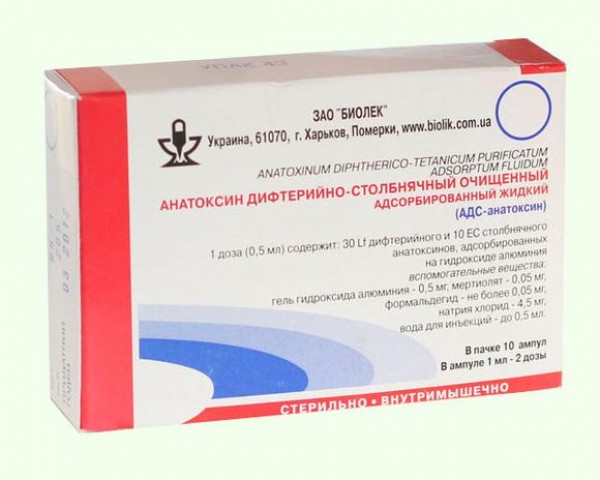
Infanrix
The most popular vaccine after DPT, it is used everywhere both from 3 months of age and adults. The vaccine is produced on the basis of a cell-free technology, without the content of merthiolate and formalin, and the reactogenicity is at the lowest level among imported drugs. The drug is sold only in pharmacies and vaccination rooms (in combination with the vaccination service), the price varies from 450 to 600 rubles (one dose). The drug is packaged in disposable syringes, complete with needles in accordance with the dosage, which has a positive effect on the convenience and speed of vaccination. The syringes are also aseptically hermetically sealed, which excludes the possibility of contamination with a non-sterile medical instrument. Doctors recommend vaccinating with this particular vaccine for babies with weakened health or allergic reactions to the components of DPT. The drug is produced in Belgium by the world famous company GlaxoSmithKline.

Infanrix Hexa
This drug is externally different from previous presence in the package of a separate suspension with the vaccine against hepatitis B. The composition of the main vaccine also contains immunogenic substances against the causative agents of poliomyelitis and hemophilic infections. The possibility of simultaneous simultaneous vaccination of pertussis, poliomyelitis, hemophilus influenzae, tetanus and diphtheria at the same time as hepatitis is very convenient - this allows you to do fewer vaccinations and does not worry about the interaction and quality of drugs.
Remember: more expensive is not always better. Infanrix Hexa costs quite an impressive amount, but the anihepatitis component in its composition is not always needed!
It is best used at the age of 6 months, when the DTP vaccination schedule coincides with the vaccination against hepatitis B. In addition, in the period from the 3rd to the 6th month of the child's life, the vaccination of hemophilus influenzae and poliomyelitis is carried out. Proper use of the vaccine with the approval of a physician can cut the number of injections needed in half. The antihepatitis component in the package is dry and mixed in one syringe with the main suspension just before injection. It is important that no other vaccines and components can be administered in this way at the same time - only the components of Infanrix Hexa. The approximate cost of the vaccine is 500–600 rubles. The package contains only one dose of the vaccine, in aseptically and sealed disposable syringe.
Tetraxim
Complex French preparation from the Sanofi pasteur company. It is also a complex vaccine containing an additional anti-poliomyelitis component. Like Infanrix it is made on the basis of cell-free technology, without merthiolate and formalin. Packaged in metered sealed syringes, one dose per package. The cost exceeds 700 rubles, however, the quality of the drug is considered the highest in comparison with other vaccines. You can be sure that after vaccination, any negative reaction.
Pentaxim
By analogy with Infanrix and Infanrix, Hexa is a variation of the usual Pentaxim. The only difference is the presence of a component that generates immunity to hemophilus influenza. There is no need to mix vaccine components, they are in finished form in one syringe. The approximate cost of the drug is 1 thousand rubles. You can injections with this drug at any time; this will not disrupt the vaccination schedule against other infections.
Final comparison
In order to simplify the question of which vaccine is better to choose, let's summarize the differences in DPT drugs. As already mentioned, the main difference between imported drugs is their production technology. Imported vaccines completely exclude merthiolate and formalin, which most often cause a negative reaction of the body to vaccination. Among such reactions can be both a compaction at the site of the vaccination for 3-4 days, and a significant increase in temperature, which will have to be brought down by antipyretics. Lack of pertussis cells (imported vaccines use parts of the cell walls, which does not bring any stress to the body, but provides the desired response immune system) is also positive factor reducing the likelihood of illness after vaccination or an allergic reaction. Despite the fact that Russian companies have already developed a non-lethal composition of the DPT vaccine and are working on acellular, the quality of these vaccines leaves much to be desired; moreover, they are not provided free of charge in hospitals.
The second advantage of imported vaccinations is their convenience: firstly, combined formulations capable of inoculating immunity immediately from the complex various infections, secondly, the dosage and packaging of drugs in syringes, which greatly simplifies the injection process and eliminates the risk of infection if the asepsis rules are not followed in the clinic. In addition, a ready-to-inject syringe and detailed instructions allows even non-qualified people to be vaccinated, if necessary. For many parents, it is important to keep the number of shots to a minimum, even at a high cost, for example, if the child does not tolerate the injections.
Whichever vaccine you decide to vaccinate in required time, make sure the authenticity of the drug and the observance of the conditions for its storage.
The most obvious and perhaps the only drawback imported vaccines is the price factor. If you are vaccinated exclusively with foreign drugs, the price of immunological procedures rises to several thousand per year. While the usual DTP is always provided by local clinics within the timeframes established by the national vaccination schedule. How many days does the temperature last after DTP and polio vaccination?  DTP vaccination, poliomyelitis, hepatitis. Vaccination with combined drugs
DTP vaccination, poliomyelitis, hepatitis. Vaccination with combined drugs
 Reaction to ads and poliomyelitis in children, possible causes
Reaction to ads and poliomyelitis in children, possible causes
Mass vaccination, about which parents and doctors are breaking spears today, was the result of epidemics of infectious diseases that took the lives of children until the middle of the last century. To stop the wave of deaths, the DPT vaccine, which has been used everywhere since the 50s of the past century, was able to.
The statistics of infant mortality in Russia and other countries before the start of mass vaccinations are approximately the same. The data is as follows:
- with diphtheria, mortality is 10%;
- with tetanus 85%;
- with whooping cough - 4% in children of the first year of life and 1% in children from 1 to 4 years old.
If we add up all these numbers, then the size of the tragedy in childhood infections becomes clear. Before the start of the massive use of vaccines, almost every child was sick with whooping cough, which in general did not improve their health. The DPT vaccine has given many children a chance at life.
Important! After the start of DPT vaccinations, mortality from these infections decreased: from pertussis - by 45%, from diphtheria - by 60%, tetanus - by 52%.
What is DTP

DTP vaccination for what? The vaccine protects against whooping cough, diphtheria and tetanus. In a newborn, maternal immunity lasts for 60 days, then the child is completely defenseless, and when infected, it will necessarily fall ill. In some countries, the first DPT vaccinations are given from 2 months, in our country - from 3 months. It is believed that there is residual immunity for another 1 month, and the child lives in conditions that reduce the risk of infection to zero.
DTP vaccination what is it? Each of the infections that the vaccine protects against is dangerous in its own way. Whooping cough causes coughing fits that can choke the infant or expose his brain to oxygen starvation... Diphtheria is dangerous due to severe intoxication and the formation of films in respiratory tract which mechanically shut off the air intake. Tetanus affects the nervous system, causing a simultaneous contraction of all skeletal muscles and hemolysis of erythrocytes.
DTP vaccination decoding - adsorbed pertussis-diphtheria-tetanus toxoid causes the formation of immunity from all three infections at once.
Toxoid or toxoid are bacterial toxins that have been soaked for a long time in a warm formalin solution. Toxoids contribute to the formation of immunity, but they are no longer capable of poisoning the body to a state of illness.
Under the name "DPT", the domestic pharmaceutical industry, namely FSUE NPO Microgen, produces a vaccine in ampoules, one ampoule contains 2 doses of the vaccine.
In some children, the pertussis component of the DPT vaccination causes a reaction; for such babies, a vaccine has been developed without pertussis toxoid - ADS and ADS-M.
Imported vaccines for the same purpose - Infanrix, Tetrakok, Pentaxim, Tetraksim. They also include an inactivated (formalin-killed) polio vaccine. Each of the vaccines has its own characteristics, but all drugs are interchangeable. Ideally, it is desirable to complete the entire vaccination schedule with one drug, but this is not always possible. All drugs, both domestic and foreign, are produced according to the same GMP standards, which are the same for all pharmaceutical companies in the world.
Comment. The GMP standard is translated as "good manufacturing practice", it is a set of all rules and regulations regarding the production of drugs, medical technology, biological and some food products... Without obtaining this standard, no pharmaceutical production can start work.
How vaccines differ from each other
There are many complaints from parents about the domestic DPT vaccine, this vaccine is considered the most dangerous. Many babies react to it with fever, moodiness, refusal to eat, crying, insomnia, and lethargy. This is an immediate reaction, it is striking and gives parents a lot of anxiety, forcing them to recklessly refuse to vaccinate.

In fact, everything is as follows. Domestic DPT, "Bubo-Kok" and the French drug "Tetrakok" are whole-cell, that is, they contain killed cells of the causative agents of whooping cough, diphtheria and tetanus. "Tetrakok" still contains formalin-treated poliomyelitis virus.
One dose (0.5 ml) of the domestic DPT vaccine contains:
- 10 billion pertussis cells
- 30 (MIE) diphtheria toxoid;
- 60 MIE tetanus.
Comment. MIE is an international immunization unit.
All these microorganisms, even killed ones, are completely foreign to the child's body. Protein and enzyme complexes, preservative merthiolate, aluminum media for absorption - all this is the first time a baby is faced with it. Naturally, this causes a violent reaction of the immune system, sometimes excessive.
The Infanrix vaccine produced by GlaxoSmithKline (Great Britain) does not contain any cells; it contains particles of cells of the pertussis pathogen and diphtheria and tetanus toxoids.
French "Pentaxim" and "Tetraxim" - also acellular vaccines, added inactivated component against poliomyelitis, as well as hemophilic bacteria. It can be used from the age of 2 months.
"Bubo-Kok" is a domestic vaccine (ZAO Kombiotech NPK), contains 10 billion killed pertussis pathogens in one dose, a component against hepatitis B has been added.
It turns out that vaccines are distinguished by the presence of cells of pertussis pathogens and additional components against other infections that can be dangerous for children from 0 to 5 years old. The treated cells of the causative agent of whooping cough Bordetella pertussis contain drugs DTP, Tetrakok and Bubo-Kok. It is they that cause a violent post-vaccination reaction.
Vaccine "Infanrix"
It is transferred much easier than cellular preparations, it contains less component parts causing various reactions. One dose (0.5 ml) contains:
- 30 MIE diphtheria toxoid;
- 40 MIE tetanus toxoid;
- 25 mcg detoxified pertussis toxin.
There is filamentous hemagglutinin, or a component of the outer membrane of bacteria, which causes adhesion of red blood cells.
Infanrix IPV additionally contains 40 units of type 1, 8 units of type 2 and 32 units of the third type of inactivated (killed) poliomyelitis virus.
"Infanrix" Hexa contains all the previous components plus the purified main antigen of the hepatitis B virus, as well as the capsular polysaccharide of hemophilic bacteria. This vaccine protects against 6 infections at the same time. The drug causes both local (edema, redness at the injection site) and general (drowsiness, fever, refusal to eat) reactions in some infants.
"Pentaxim"

The Pentaxim vaccine of the French manufacturer Sanofi Aventis Pasteur is available in ready-to-use disposable syringes. One dose (all the same 0.5 ml) contains:
- 30 MIE of diphtheria toxoid;
- 40 MIE tetanus;
- 25 mcg pertussis toxoid;
- 25 μg filamentous hemagglutinin;
- 40 units of type 1, 8 units of the second and 32 units of type 3 of the polio virus.
Aluminum hydroxide, Hanks medium (mixture of amino acids), phenoxyethanol (formaldehyde derivative), sodium hydroxide or acetic acid are used as auxiliary substances.
It works against hemophilus influenza b, which causes septicemia (spread in the bloodstream) and meningitis (inflammation of the lining of the brain). As auxiliary substances, sucrose and trometamol are used - a regulator of the balance of water and electrolytes.
"Tetraxim"

Manufacturer - Sanofi Pasteur, France. Protects against diphtheria, tetanus, whooping cough and polio. The drug is acellular, contains only toxoids and killed poliomyelitis viruses of three main types. Available in 0.5 ml disposable syringes with a fixed needle. Can be used from the age of 3 months. You can give other vaccines on the same day, but the syringes must be different, as well as the injection sites.
Aluminum hydroxide, formaldehyde (for disinfecting action), phenoxyethanol, a preservative and antiseptic, are used as auxiliary agents.
"Bubo - Kok"

Available in 0.5 ml ampoules, 10 ampoules in a package. Used to vaccinate children from 3 months to 4 years old. It contains diphtheria and tetanus toxoid, killed Bordetella cells, antigen to the hepatitis B virus. Merthiolate is used as a preservative.
Merthiolate or thiomersal is a mercury compound used as an antiseptic. Concerns about toxicity are unfounded, since mercury vapor is poisonous, not its compounds.
All children vaccinated with the same batch are monitored. If 1% have local reactions, and 4% have general reactions, then the use of this series is stopped.
Allowed the simultaneous introduction of other vaccines according to the vaccination schedule.
Vaccination schedule

Any DPT vaccine is administered on a national schedule at the following times:
- the first at 3 months;
- the second after 30 or 45 days - at 4 or 5 months;
- third at 6 months;
- fourth in a year and a half.
Such intervals are necessary for full-fledged formation cellular response. Revaccination is performed at the age of 7 and 14 so that by the time of graduation from school the teenager is completely protected.
This vaccination schedule is used all over the world. Infections know no borders or governments, they have their own laws. All over the world, the first vaccination against diphtheria, tetanus, pertussis and poliomyelitis is performed at 2-3 months, and then it is repeated at intervals of one and a half months, and so on. Only the names and the composition change a little, but the principles are the same for everyone. When vaccinated, the epidemiological situation is assessed first of all.
Formed by the age of 14, immunity is "enough" for 10 years. Then it is desirable to repeat the introduction of the vaccine every subsequent 10 years.
How to prepare your child

Preparation for the DPT vaccination begins at home. The main condition is that the baby must be healthy on the day of vaccination. 3-4 days before the first vaccination at 3 months, he should not have a fever, runny nose, indigestion, prolonged anxiety. Everything should go as usual. The visiting nurse should explain in detail how to prepare for the DPT vaccination.
Important! Parents who are afraid of vaccinations should be aware that a baby can face any infection every minute - on the street, in transport, during a visit. People live in the world of microbes, and who will take it depends on the strength and fitness of the body.
Facing infections is inevitable; no one lives in a sterile world. Vaccinations offer a lighter version of this encounter when the disease-causing properties of the pathogens are drastically weakened.
Nothing new should be added to the child's nutrition on the eve of vaccination. If the baby is eating breast milk, then mom shouldn't try anything new either. Each New Product Is a potential allergen that should be avoided.
Directly on the day of vaccination, the baby needs to be well watered, but you cannot feed it. Some children react with nausea or a single vomiting, it is not necessary to create conditions for this.
The kid needs to be dressed for the weather so as not to sweat. An overheated baby "picks up" more easily respiratory infections that are present everywhere.
In the morning, you need to make sure that the baby has a chair. Emptying the intestines at the time of vaccination is an unnecessary nuisance.
In each vaccination room there is an anti-shock cabinet. It stores the medicines needed to stop a severe allergic reaction - if it develops. After the injection, you need to be in the clinic or nearby for 40 minutes in order to get help if necessary. If after 30-40 minutes there is no allergic reaction, then it does not develop in the future.
Contraindications
The DPT vaccine is a complex of complex compounds, each of which can cause unwanted reactions... Therefore, there are contraindications for manipulation, which can be temporary or permanent. The list of contraindications was approved in the guidelines of the Ministry of Health of the Russian Federation on January 9, 2002.
Temporary contraindications - respiratory infections and digestive disorders, the treatment of which may take no more than a week. If at the time of vaccination the baby is sick, then the date is postponed until recovery, and the entire schedule is shifted accordingly. The same applies to all subsequent periods of drug administration.
Permanent contraindications for DTP vaccination:
- Progressive diseases of the nervous system - hereditary or acquired. In some cases, the drug is administered without a pertussis component, the decision is made by a pediatric neurologist.
- Afebrile convulsions (not with a high fever). Such a child needs examination to find out the cause of the seizures.
- All forms of immunodeficiency.



In the event that the epidemic situation is threatening, and the baby had a pronounced reaction to the previous administration, then they are vaccinated with ADS and ADS-M preparations (without the pertussis component) under the protection of a double (one day before and after administration) use of prednisolone (steroid hormone). A short injection of the hormone helps to smooth out the allergic reaction in children to the DPT vaccine.
Post-vaccination reactions
Side effects in children for DPT vaccination are divided into post-vaccination reactions and complications.

Post-vaccination reactions are local and general. Local reactions to the DPT vaccine are swelling and redness at the injection site.
Comment. Babies are vaccinated with DPT in the thigh so that the drug gets into the muscle, forms a depot there and is slowly absorbed into the bloodstream. Older children are allowed an injection into the deltoid muscle of the shoulder. Inject the vaccine under the skin or into adipose tissue it is forbidden.
A normal local reaction is redness up to 8 cm.Some parents complain that a lump-shaped seal has formed at the injection site, and blame nurse in inability.
In fact, the lump is formed by aluminum oxide, which is for this purpose introduced into the DPT vaccine. It is an adsorbent that increases immunogenicity. There should be inflammation at the injection site of the vaccine, it is necessary so that the child's immune system gets to know the pathogens as closely as possible. This is a depot of the drug - the DPT vaccine, which is necessary for the formation of an expanded cellular response.
A lump does not form in all children after DPT vaccination. This does not mean that there is no response from the body, it is different for everyone.
The severity of the overall reaction to the vaccine differs in terms of the rise in temperature:
- up to 37.5 0 С - light;
- from 37.5 to 38.5 0 С - moderate;
- above 38.5 0 С - severe.
Post-vaccination reaction after vaccination is considered a normal response to the introduction of foreign substances. It is necessary to consult a doctor if the temperature after the DPT vaccine rises above 38.5 0 С, as well as in case of convulsions, prolonged shrill screaming, severe anxiety or constant sleepiness, complete refusal to eat, repeated vomiting or diarrhea. Consultation is also required if the redness at the injection site of the vaccine is more than 8 cm.
Complications
Side effects of DPT vaccination are considered complications in such cases:
- suppuration at the injection site of the vaccine, requiring surgical treatment;
- allergic rash;
- Quincke's edema or shock after a vaccine - conditions that are life-threatening without medical intervention;
- convulsions without fever, which indicate damage to the central nervous system.
Such complications from DPT vaccines are rare, and there are only three reasons for this: a sick child, violation of the rules for storing vaccines and non-compliance with the rules of administration. The consequences of vaccination in children are when two or three of these factors coincide.
Comment. Word of mouth and online reviews will help you quickly find out the truth about a particular hospital, as well as about the DPT vaccine series.
The incidence of real complications after DPT is on average 1 in 3 million vaccinated children.
Portability at different times

The reaction to the first vaccinations at three months can be violent but short. Usually, all post-vaccination reactions to the vaccine last no more than 3 days. If the child is worried about something after this, then most likely a respiratory infection has joined.
How calmly the second DPT is transferred depends largely on the reaction to the first. In some babies, behavior, sleep and appetite do not change at all.
For some parents and children, the third DPT is becoming a problem. By the 6th month, the response of the immune system is already quite active, and the child can have high fever, diarrhea, vomiting, refusal to eat and general malaise.
The reaction most often occurs to DPT vaccines containing the cellular pertussis component. Acellular vaccines have far fewer side effects.
Revaccination of DPT is transferred in almost the same way as previous administrations. Normally, the severity of the reaction from injection to injection increases, but very slightly. Often this weighting is noticed only by a wary mother.
How revaccination is tolerated depends on the drug itself. There is one general rule: if the baby reacted badly to the drug, then it is not necessary to repeat its introduction, it is better to replace it with an analogue. All vaccines contain necessary components but carry over differently.
Do it or not
Vaccination, the consequences of which seem terrible to parents - real flowers compared to expanded clinical picture severe infections... The death of babies from infections is an irreparable tragedy.
Every child who comes into this world goes through natural selection. It is not a fact that without vaccinations and vaccines, he will withstand it. How to prepare your child for the DPT vaccine? Carefully observe his behavior, instill only healthy people and do not interfere with the development of the immune response by adding new food and drink.
Post-vaccination symptoms are only echoes of the drama that unfolded in families less than a century ago. In backward countries, children still die from tetanus and diphtheria, the natural foci of which have not disappeared anywhere. The causative agents of deadly infections simply cannot "lift their heads" due to mass vaccinations.
It is very difficult to observe a sick baby. But it is much worse to part with him forever because of his own unreasonable behavior.
Watch the video - Komarovsky's answers about the DPT vaccine:
The DPT vaccine is one of the first in a baby's life. The child receives immunity from three deadly diseases at once - tetanus, whooping cough and diphtheria. These are three compatible vaccines that can be given at the same time. As a result, you do not need to take the baby to the clinic three times and subject it to injection tests each time - just once is enough. Children take one injection much easier than three.
Vaccination schedule
When is the DTP vaccine given? According to national calendar children should be vaccinated 4 times up to one and a half years from DPT. The first vaccination is at three months. The second vaccination is given 41-45 days later. Further vaccination is carried out at 6 and 18 months.
- 3 months - first dose
- 4.5 months - repeated dose;
- 6 months - third dose;
- 1.5 years is the last dose.
The next stage is revaccination to maintain the mass of antibodies at the required level. Children are revaccinated at the age of 7, and then at the age of 14. After that, immunologists advise to carry out revaccination of DPT independently every ten years without the pertussis component: this disease is not dangerous for adults.
Why do adults need the vaccine every ten years? Because over time, the level of antigens in the blood decreases and the immune response weakens, therefore, an adult can become infected with viruses. However, a previously vaccinated person carries the virus much more easily than an unvaccinated person.
It is very important to endure correct timing vaccinations: three vaccinations with an interval of one and a half months. It is in this sequence and concentration of the drug that a stable immune response to viruses is developed. If for some reason the child has not been immunized before the age of 4, he is given a drug without the pertussis group - ADS.
Important! Vaccination can be postponed due to ill health, however, it is impossible to vaccinate earlier than the deadline set by the rules!
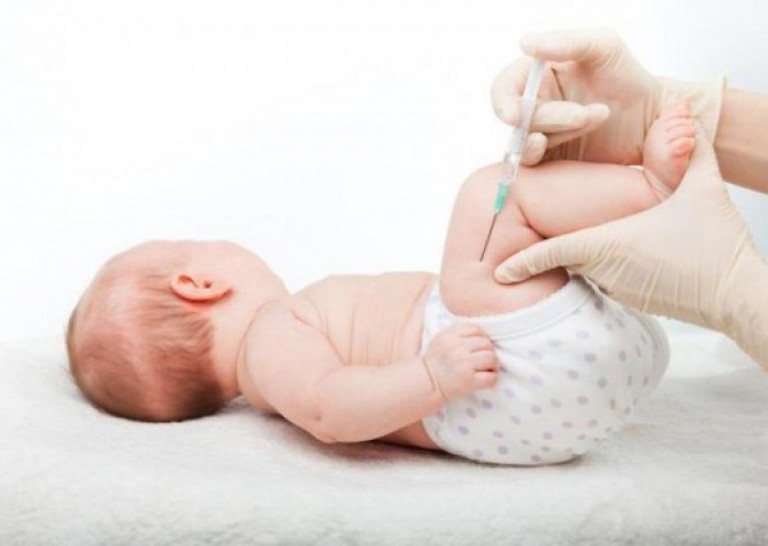
Immunizing children
Why is the first DPT vaccine given at three months? During intrauterine formation, the fetus received the necessary substances from the mother's body through the umbilical cord. However, after 60-65 days, the effectiveness of antibodies decreases, and the baby is vaccinated against deadly diseases.
After 45-50 days, children should receive a second dose of DPT vaccine. Usually, the reaction to the first vaccination is not pronounced, babies react to the drug without complications. The second vaccination in many babies causes complications, and the reaction is extremely severe.
A more severe reaction to the second injection is caused by the presence of antibodies to these viruses: the body begins to fight against the introduction of the inactivated strain. That is, the immune response began to take effect. The third injection is also tolerated in a more severe form for the same reason. After the third injection, the immune response is considered to be formed.
Important! If the second DPT vaccination was missed for any reason, it is necessary to vaccinate the child later. It is impossible not to give a second injection at all.
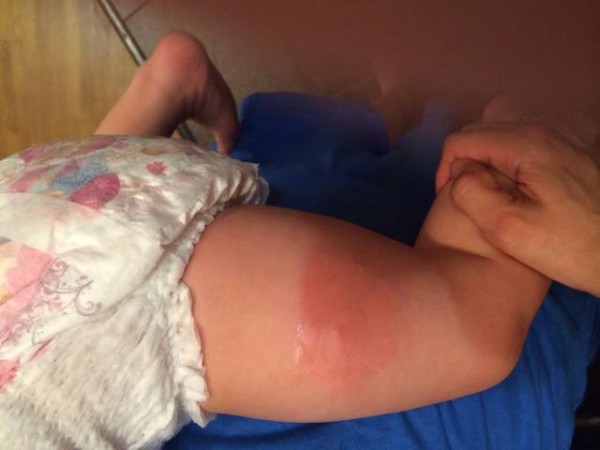
Complications
If the child develops adverse symptoms after the second vaccine, another drug is used for the third time. It is important to save the baby from unnecessary torment. A negative response to DPT can be caused by the pertussis component, therefore, subsequent vaccination is carried out without it.
The body's response to inactivated whooping cough can be expressed in a rise in temperature to 39C and above, disorders of the nervous system (moods, sleep disturbances, lethargy or irritability). A local reaction to DPT inoculation in the form of redness and soreness at the puncture point is caused by aluminum hydroxide, which is involved in the vaccine composition as an immune response enhancer.
It is important to distinguish between side effects from drug administration and complications. Complications are a severely impaired reaction to DPT. Complications after the vaccine include:
- severe laryngeal edema;
- hives;
- convulsions.
These complications can be caused by an allergic reaction of the body to the components of the drug / serum. In severe allergic changes, revaccination is not performed or the drug is replaced with an analogue (everything is decided by the doctor).
Side effects
Side effects are temporary and cannot be treated. Adverse Reactions the vaccine can be local in nature (redness of the vaccination site, itching, induration) and general nature. To manifestations general relate:
- tearfulness and small mental deviations;
- upset gastrointestinal tract - lack of appetite, constipation / diarrhea, nausea;
- slight limp due to soreness at the injection site;
- coughing due to the influence of the whooping cough component.
There may also be a slight hyperthermia within 38C. At pain pain relieving ointments can be used in the baby's leg on the advice of a pediatrician. You should create an atmosphere of relaxation for the child, exclude any activity - walks, water procedures.
If the baby tolerates vaccination in a satisfactory form, you can take a short walk the next day and bathe the baby in the bathroom. The injection site should not be rubbed with a washcloth and injured. If there is a lot of redness and swelling, the baby should be taken to the clinic.
Advice. If the reaction is expressed by hyperthermia, you should give the baby an antipyretic. At low temperature put candles with paracetamol rectally, with high temperature give syrup with paracetamol or other means.
Preparing for immunization
To avoid Negative consequences after vaccinations, you should properly prepare for this procedure. The basic rules of preparation include:
- examination of the child and delivery of tests;
- taking antihistamines (antiallergic) drugs;
- light breakfast before vaccination.
The child must be completely healthy before vaccination. This condition must be ascertained by a pediatrician. It is desirable to pass required analyzes to confirm full health baby.
Is it possible to do DTP for teething and runny nose for children under one year old
DTP (adsorbed diphtheria-tetanus pertussis vaccine) - This is a combined vaccine, the action of which is directed against three infections at once: diphtheria, pertussis, tetanus. Children are being vaccinated against these dangerous diseases at the age of three months. To develop immunity, it is necessary to administer the DPT vaccine three times. Vaccinations against these diseases are carried out in almost all countries of our planet. Nevertheless, the DPT vaccine is considered the most dangerous in the world due to the high percentage side effects and complications, as well, a large number allergic reactions in children.
What does DPT protect from?
Whooping cough, diphtheria and tetanus are dangerous infectious diseases that can lead to grave consequences for human body... These diseases are especially difficult for children. Mortality from diphtheria reaches 25%, from tetanus - 90%. Even if the disease was defeated, the consequences of them can remain for a lifetime - chronic cough, impaired functioning of the respiratory and nervous systems.
What is DPT vaccine?
DTP - This is a domestic vaccine that is administered to children under the age of 4 years. For revaccination after 4 years, foreign drugs are often used, which are officially registered in our country - Infanrix and Tetrakok. DTP and tetracoccus are similar in composition - they consist of killed cells of infectious agents. These vaccines are also called whole cell vaccines. Infanrix differs from DTP in that it is an acellular vaccine. This vaccine contains small particles of pertussis microorganisms and diphtheria and tetanus toxoids. Infanrix causes a less violent reaction of the body than DPT and tetracoccus, and causes fewer complications.
When is the DTP vaccine necessary?
There is a calendar of vaccinations, which the doctors of our country adhere to. The first DPT vaccine is given to children at the age of 3 months, the next at 6 months. At the age of 18 months, the child needs another DPT revaccination. Only after three times of vaccination do children develop immunity against diseases. If the first DPT vaccination is given to a child not at 3 months, but later, then the interval between the first two vaccinations is reduced to 1.5 months, and revaccination is carried out 12 months after the first vaccination. The next revaccination is carried out only against tetanus and diphtheria in children at the age of 7 and 14 years.
How is vaccination done?The DPT vaccine is administered intramuscularly. Up to 1.5 years, the vaccine is administered in the thigh, for children older - in the shoulder. All preparations are a cloudy liquid, which is shaken thoroughly before administration. If the capsule contains lumps or flakes that do not dissolve, then this vaccine should not be administered.
Reaction to DPT vaccine
After the introduction of the DPT vaccine, the child may have a response. The reaction is local and general. A local reaction manifests itself in the form of redness and induration at the injection site. The general reaction can be expressed elevated temperature and indisposition. If, after the DPT vaccination, the child's body temperature has risen to 40 degrees, then the vaccine should be stopped and other drugs should be used, for example, pentaxim (French vaccine). Almost all complications after DPT vaccination are noticeable in the first few hours after  vaccination. Any complications after DPT are associated with individual characteristics child's body... TO dangerous consequences after DPT include a sharp increase in temperature, disorders of the nervous system, developmental delay.
vaccination. Any complications after DPT are associated with individual characteristics child's body... TO dangerous consequences after DPT include a sharp increase in temperature, disorders of the nervous system, developmental delay.
If your child has a negative reaction to the drug, see your doctor right away.
ContraindicationsDTP vaccination is contraindicated in children with changes in nervous system, diseases of the kidneys, heart, liver, as well as those suffering from infectious diseases.
