The procedure for prescribing and prescribing medications. Prescription Forms
"On approval of the order of appointment and discharging drugs, as well as forms of prescription forms for medicinal products, the procedure for registration of these forms, their accounting and storage "
In the current latest edition from 30.06.2015 N 386н
With changes and additions that entered into force on 01.01.2016
The beginning of the revision: 01/01/2016
Registered in the Ministry of Justice of the Russian Federation on June 25, 2013 N 28883
With all Appendices - order (rules), form-forms
In accordance with paragraph 16 of part 2 of article 14 Federal law of November 21, 2011 N 323-FZ "On the basics of health protection of citizens in Russian Federation"and paragraph 5.2.179 of the Regulation on the Ministry of Health of the Russian Federation, approved by the Government of the Russian Federation of June 19, 2012 N 608, approves the procedure for prescribing and prescribing drugs in accordance with Appendix No. 1; forms of prescription forms in accordance with Appendix No. 2; the procedure for issuing prescription forms, their accounting and storage in accordance with Appendix No. 3.
- Appendix N 1. The procedure for prescribing and prescribing medications
- I. General Provisions
- II. Prescribing drugs when providing medical care in stationary conditions
- III. Prescribing and prescribing drugs in the provision of primary health care, emergency medical care and palliative care
- IV. Prescribing and prescribing medicinal products to citizens who have the right to free receipt drugs or receiving drugs at a discount, as part of their primary health care
- Appendix N 1. Maximum permissible number of individual narcotic and psychotropic medicinal products for prescription per prescription
- Appendix N 2. Recommended number of individual medicinal products for prescription per prescription
- Appendix N 3. Acceptable prescription abbreviations
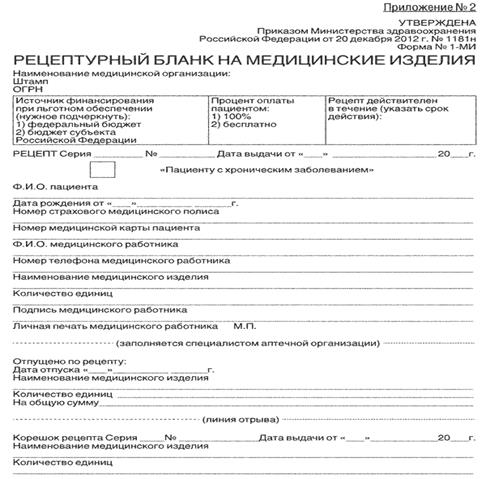
- Appendix N 2
- Prescription form (Form N 148-1 / y-88, OKUD form code 3108805)
- Prescription form (Form N 107-1 / y)
- Prescription form (Form N 148-1 / u-04 (l), form code according to OKUD 3108805)
- Prescription form (Form N 148-1 / y-06 (l), form code according to OKUD 3108805)
- Appendix N 3. The procedure for registration of prescription forms for medicinal products, their accounting and storage
- I. Registration of prescription forms
- II. Accounting for prescription forms
- III. Storage of prescription forms
This procedure governs issues prescribing and prescribing medicinal products in the provision of medical care in medical organizations, other organizations providing medical care, and individual entrepreneurs carrying out medical activities.
It is carried out by the attending physician, paramedic, midwife if they are entrusted with the powers of the attending physician in the manner prescribed by the order of the Ministry of Health and Social Development of the Russian Federation of March 23, 2012 N 252n medical organization when organizing the provision of primary health care and emergency medical care of individual functions of the attending physician for the direct provision of medical care to the patient during the period of observation and treatment, including the appointment and use of drugs, including narcotic drugs and psychotropic drugs " (registered by the Ministry of Justice of the Russian Federation on April 28, 2012, registration N 23971), individual entrepreneurs engaged in medical activities.
Medical workers write prescriptions for medicinal products under their own signature. Prescribing and prescribing of medicinal products is carried out by a medical worker under the international nonproprietary name, and in his absence - by the grouping name. In the absence of an international non-proprietary name and a grouping name of a medicinal product, the medicinal product is prescribed and prescribed by a medical professional by the trade name. In the presence of medical indications(individual intolerance, for health reasons), according to the decision of the medical commission of the medical organization, the prescription and prescription of drugs is carried out: not included in the standards of medical care; by trade names. The decision of the medical commission of the medical organization is recorded in the patient's medical documents and the journal of the medical commission. Medical workers prescribe and prescribe medications to be manufactured and dispensed by pharmacy organizations (individually manufactured medicinal products).
Prescribing and prescribing medications when providing medical care in inpatient conditions, it is carried out under the international non-proprietary, grouping or trade name. A prescription written out in violation of the requirements established by this Procedure is considered invalid. Information about the prescribed and prescribed medicinal product (name of the medicinal product, single dose, method and frequency of administration or administration, duration of the course, justification for prescribing the medicinal product) are indicated in the patient's medical record. A prescription for a drug is written in the name of the patient for whom the drug is intended. A prescription for a drug can be obtained by the patient or his legal representative. The fact of issuing a prescription for a medicinal product to a legal representative is recorded in the patient's medical record.
Full text version- in the attached file. The file is indexed (the "Properties" are filled in as expediently as possible), which makes it more convenient when using and searching on a computer, provided with informative headers and footers. The file is economical: it is minimal in weight and contains only text.
Order of the Ministry of Health of the Russian Federation No. 110 of 12.02.07 approved 4 forms of prescription forms:
A special prescription form with a serial number and degree of protection.
RB form No. 148 - 1 / U - 88
RB No. 148 - 1 / U - 04 (l)
RB No. 148 - 1 / U - 06 (l)
No. 107 - 1 / U
Mandatory and basic details of special prescription forms:
MU stamp indicating its name, address and telephone number
Full name of the patient
Patient's age (number full years)
Full name of the doctor
Prescription date
Drug name, quantity
Method of using drugs
Doctor's signature and personal seal
Prescription validity
Special RB made on paper color pink with watermarks. The prescription must be written in the hand of the doctor who signed it. On the form of this form, NS and PVII lists are written out, the number of NS and PV prescribed in the prescription (ampoules, tablets, capsules, etc.) must be indicated in words.
Additional details of the special RB:
Serial number
Outpatient medical record number (medical history)
Leave of the chief physician or his deputy, who is responsible for the appointment of these drugs
Round stamp of LPU
On the form of this form, it is allowed to write out only one name of the drug. The validity period is 5 days.
RB form No. 148 - 1 / U - 88 intended for prescribing:
Medicinal prescriptions of individual manufacture, containing NS or PV of Schedule II and other pharmacologically active substances in a dose not exceeding WFD and provided that this combined drug is not NS / PV-II;
PC List III;
Schedule IV precursors;
Potent substances subject to PKU;
Poisonous substances subject to PKU;
List-A drugs (Apomorphine hydrochloride, Atropinosulfate, Homatropin hydrochloride, Dicalin, Silver nitrate), List-B (Pachikarpina hydroiodite);
Ethyl alcohols;
Medical antiseptic solution;
Anabolic steroid;
Coaxil;
Zaldiar;
Butarfanol.
Additional details of form No. 148 - 1 / U - 88:
Serial number
Patient's address / place of residence, medical card number
Medical facility stamp
This RB is valid for 10 days. It is allowed to write out 1 name of the drug on it.
RB form No. 107 - 1 / U ... This form is intended for prescribing all other drugs, including PV that are not subject to PKU. Validity period - 2 months.
The maximum permissible amount of drugs for prescribing for 1 prescription and the case of their increase (rates of one-time dispensing).
Codeine, codeine phosphate, powder form, release rate 2.0 dg
Combined drugs containing codeine or codeine phosphate not more than 2.0 dg
Pachicorpina hydrochloride (iodide) - powder, release rate 1.2 g
Derivatives of barbituric acid: phenoborbital - a form of release in tablets of 50 mg and 10 mg, the norm is 10-12 tab.
Ethyl morphine hydrochloride (Dionide) - powder, release rate 2.0 dg
Ephedrine hydrochloride - powder, release rate 6.0 dg
Ethyl alcohol mixed with other ingredients no more than 50.0; pure ethyl alcohol up to 50.0 according to recipes with the inscription "for applying compresses", "for skin treatment"
In cases of an increase in the maximum permissible quantities of drugs by 1 prescription:
Dianine or ethyl morphine hydrochloride in eye drops and ointments can increase up to 1.0 if the prescription has an inscription “by special purpose", Certified by the signature and personal seal of the doctor.
Derivatives of barbituric acid, ephedrine - in pure form and in a mixture with other medicinal products to patients, due to a chronic illness, a course of treatment for up to 1 month, provided that the prescription has an inscription "for special purposes", certified by the signature and personal seal of the doctor.
Ethyl alcohol for chronic patients for individual drug prescription up to 100.0 in a mixture and pure form, if the prescription has an inscription "for special purposes", certified by the signature and personal seal of the doctor.
At present, by order of the Ministry of Health No. 110 dated February 12, 2007 (as amended on February 26, 2013) "On the procedure for prescribing and prescribing medicines, products medical purpose and specialized products health food"And No. 54n dated 01.08.2012, No. 1175n dated 20.12.2012) approved following forms prescription forms:
1) Form "Special prescription form for narcotic drug and psychotropic substance ”107 / y-NP;
2) Form No. 148 -1 / y-88 "Prescription form";
3) Form No. 107 -1 / y "Prescription form";
4) Form No. 148 -1 / y - 04 (l) "Recipe";
5) Form No. 148 –1 / y - 06 (l) "Recipe".
1. Form "Special prescription form for a narcotic drug and psychotropic substance" according to the instructions to the order №110 and №1175н "prescription forms are protected printing products of level" B ", made on pink paper measuring 10 cm x 15 cm, must have a series and number." On the prescription form of this sample, narcotic drugs and psychotropic substances are prescribed, included in List II of the List of narcotic drugs, psychotropic substances and their precursors subject to control in the Russian Federation (according to the Decree of the Government of the Russian Federation No. 681 of June 30, 1998 (as amended on 02.07.2015 )).
The prescription form is filled in legibly and clearly. The form is stamped with a medical organization (indicating full name medical organization, its address and telephone), the date of the prescription. The prescription fully indicates the surname, name, patronymic of the patient, age (full years). The series and number of the mandatory policy must be indicated health insurance, "Case history No.", or "No. of the medical card" of the patient, or the history of the child's development, medical history. In addition, the surname, name and patronymic of the doctor are fully indicated. In the line "Rp:" on Latin the name of the narcotic drug, its dosage, quantity and method of administration are indicated. The prescription is signed by the doctor who wrote this prescription, after which it is certified by the doctor's personal seal. Additionally, it is certified by the round seal of the healthcare facility and signed by the chief physician or his deputy.
Only one name is allowed on one prescription form. medicinal product and no corrections are allowed. The amount of prescribed drugs is indicated in words. The method of taking drugs is indicated in Russian. The recipe stays in pharmacy organization for PKU.
2. Form No. 148 -1 / y-88 "Prescription form" has a series and a number. In addition, it must contain the following details: the address or number of the patient's medical record, the stamp of the medical institution “For prescriptions”, full name. the patient and the doctor completely. For free and preferential vacation, the prescription is issued in two copies. Psychotropic substances are prescribed on this prescription form List III The list of narcotic drugs, psychotropic substances and their precursors subject to control in the Russian Federation (according to the Decree of the Government of the Russian Federation No. 681 dated June 30, 1998 (as amended on 07/02/2015)), as well as other drugs that are subject to quantitative accounting and anabolic steroids.
On one prescription form, it is allowed to prescribe only one name of the medicinal product, and with back side prescription, a note is made about who prepared, checked and dispensed the drug. The prescription remains in the pharmacy organization for subject-quantitative accounting Appendix 2.
3. Form No. 107 -1 / y "Prescription form". All medicines are prescribed on this prescription form, with the exception of those prescribed on the prescription form No. 148 -1 / y - 88 and a special prescription form for a narcotic drug and a psychotropic substance. The prescription is signed by the doctor and certified by his personal seal.
No more than 3 names of medicines are written out on one prescription form, and corrections are also not allowed. Ethanol are written out on a separate form and additionally certified by the seal of the LPU "For prescriptions". Annex 1.
4. Form No. 148 -1 / y -04 "Recipe" and Form No. 148 -1 / y-06 "Recipe" are intended for prescribing medicines on preferential terms (free or at a discount), and the form №148 -1 / -06 is drawn up using computer technology. On the prescription form of the listed forms, medicines, medical products and specialized medical food products for disabled children are prescribed.
Prescription form is issued in 3 copies, having a single series and number, while the prescription is signed by a doctor (paramedic) and certified by his personal seal. When dispensing a medicinal product in pharmacy on the prescription form, information on actually dispensed drugs is indicated, and the date of release is stamped. This prescription form there is a tear line separating the form and the root, which is issued to the patient. In this case, a mark is made on the spine about the name of the drug, dosage, quantity, method of application.