Influvac vaccine composition. Influvac ® (subunit inactivated influenza vaccine) (Influvac ®)
Vaccination - The best way improve immunity and stay healthy even during exacerbation of situations with acute respiratory viral infections. One of the most popular and quality medicines for influenza is the Influvac vaccine.
This vaccine is produced in the Netherlands. Influvac is used as prophylactic agent from the flu. Immunity to viruses is formed within two weeks after injection and lasts up to 1 year.
Composition and form of release
The drug is sold in dosage form - in disposable syringes of 0.5 ml. This makes the vaccination process safe. The package can contain from 1 to 10 syringes.
The active substances of the agent are viral strains of influenza. The composition of antigens in the vaccine is updated annually. The manufacturer is guided by the latest recommendations from the World Health Organization (WHO). The organization's reports contain the most actual information about dangerous strains on the territory. This collaboration makes it possible to create an effective and high quality flu remedy.
The classic composition includes the two most dangerous strains of the influenza A. Another strain changes every year.
The auxiliary elements in the composition include:
- sodium;
- potassium;
- calcium;
- magnesium.
From the video you can understand the principle of action of the vaccine "Influvac" on the human body. Active substances, which are part of the drug, make the immune system work better and respond quickly to viruses.
Who needs the vaccine?
 Influvac vaccine can be used for children starting from six months. Such a means of prevention is suitable for everyone. But there are certain groups of people who are especially susceptible to disease. Vaccination in such cases is simply necessary:
Influvac vaccine can be used for children starting from six months. Such a means of prevention is suitable for everyone. But there are certain groups of people who are especially susceptible to disease. Vaccination in such cases is simply necessary:
- elderly people over 65;
- with diseases of the respiratory organs;
- people who have problems with the heart, blood vessels and blood flow;
- with chronic kidney disease;
- in the presence of diabetes mellitus;
- with immunodeficiency diseases - HIV, blood diseases (malignant);
- when taking immunosuppressants, cytostatics, high doses of corticosteroids, as well as when undergoing radiation therapy;
- children from 6 months to 18 years old - when taking acetylsalicylic acid, the risk of Reye's syndrome and susceptibility to influenza infections increases;
- starting from the second trimester, it can also be used for the prevention of pregnant women - without reference to the date, women who are in high-risk groups are vaccinated.
Contraindications
Like most drugs, this vaccine has contraindications. You should immediately familiarize yourself with them so that, instead of protecting the body, they do not aggravate the situation:
- Allergic reaction. It can be both hypersensitivity and individual intolerance to any components of the vaccine (for example, chicken protein can act as an allergen).
- If previous injections with similar vaccines caused fever or allergies, then you should abandon this drug.
- The procedure should be postponed if an exacerbation of chronic diseases has occurred.
During mild forms of ARVI, and other diseases (even in the acute stage), it is recommended to apply the vaccine after establishing normal temperature body.
Vaccine instructions
 The dosage of the drug differs depending on age:
The dosage of the drug differs depending on age:
- from 6 months to 3 years, 0.25 ml is administered;
- starting from 3 years, the dosage rises to 0.5 ml.
For children who have not previously been vaccinated, it is better to have two injections. The interval between each of them should be 4 weeks.
Prevention should be carried out every fall. Immunity is formed within two weeks after the injection. And the developed protective barrier is enough for the whole year.
The drug is administered intramuscularly or deeply subcutaneously.
Intravenous use of the Influvac vaccine is prohibited.
Before administering the drug, it should be heated to room temperature. The syringe is shaken, the protective cap is removed from the needle, air is removed from the syringe.
Are there complications?
The vaccine has been used over 23 million times in five years. At the same time, few cases in which side effects appeared.
Reported complications:
- Local nature - swelling, redness, pain at the injection site, inflammation of the lymph nodes.
- General weakness, headaches, sweating, fever, muscles and joints may hurt. Usually, such phenomena disappear on their own, after a few days.
- Neuralgia, paresthesia, seizures, and thrombocytopenia may appear.
- Sometimes observed neurological disorders, vasculitis. There are also short-term failures in the work of the kidneys.
- A rare exception is the occurrence of shock due to an allergic reaction of the body.
Analogs
There are several vaccines similar to Influvac. Of the Russian ones, the following can be distinguished:
- Pandeflu is sold in metered ampoules. The drug is injected into the area of the deltoid muscle.
- "Ultravac" - must be mixed with a solvent. Inserted into the sinuses using a special spray nozzle.
There are also several popular medicines of imported origin:
- "Begrivak" - produced in France. The peculiarity is that the drug contains not only surface, but also internal antigens. Therefore, there are no toxins in such medicines.
- "Agrippal S1" - this vaccine is produced in factories in Italy and Spain. The drug stimulates immunity only to three of the constituent strains or very close to it. Therefore, it is ineffective against others. Also, it cannot be used during the period of illness. And the vaccination itself should be carried out in a hospital.
Influvac - vaccine High Quality... Every year it is being improved and adapted to the requirements of the current epidemiological situation in Eurasia. Therefore, using "Influvac", you can not be afraid of the flu and its complications.
The approach of cold weather is marked by an increase in the risk of contracting the flu. By the way, a hundred years ago this disease became fatal for hundreds of thousands of people. Today percentage deaths from it is not too large, however, many patients have to deal with severe complications.
Real reviews and discussions


Fortunately, modern doctors know not only how to treat this infection, but also how to prevent its development. One of the most effective options prevention can be vaccination... In particular, the use of the influenza vaccine Influvac is considered relevant, which should be discussed separately.
Vaccination - best prevention flu
If in the past (before the invention of vaccination) people did not know how to protect themselves from influenza, today they face another problem - which vaccine to choose for the most effective prevention among the many options on offer?
It never happens that a virus remains the same over the years: it is constantly changing and undergoing various mutations, new strains of it appear. Medical specialists working at WHO, every year before the epidemic, predict which types of infection will have to be dealt with. Based on this, vaccine manufacturers are making new drugs to combat suspected viruses.
Influenza vaccine Influvac contains surface antigens of killed viral strains:
- Chicken embryos are used for their cultivation.
- The next stage is selection.
- Viruses are inactivated (for which formaldehyde is used).
- Surface particles with antigens are separated from them.
- The rest is thoroughly cleaned.
As a result, a subunit vaccine is obtained containing neuraminidase as well as hemagglutinin. The composition includes purified viral antigens of several types and, of course, is updated every year (the instructions indicate the season - this is especially worth paying attention to). As with other vaccines, the drug lasts for a year, and therefore every time before an epidemic begins, it is necessary to be vaccinated again.
The country of origin is the Netherlands. Since the late 1980s, the vaccine has been officially registered and used in our country.
The vaccine is valid throughout the year
Along with viral particles, Influvac consists of:
- sucrose sodium citrate;
- polysorbate;
- potassium and sodium chloride;
- formaldehyde;
- calcium chloride dihydrate;
- sodium phosphate dihydrate;
- chicken protein.
All of these help to strengthen the immune system and provide protection against infectious attacks.
Indications for vaccination
It is advisable to get vaccinated against influenza for everyone who cares about their health and intends to avoid infection in the upcoming epidemic... However, there are categories of people for whom vaccination is highly recommended:
- persons who often suffer from ARVI;
- cores;
- people over 60 years old;
- diabetics;
- people with weak immunity(no matter for what reasons it has become weaker);
- children who often take aspirin (in case of infection with the flu, they may develop Reye's syndrome, which is dangerous to the vital activity of the liver);
- pregnant women (usually the second half of the term).
As for contraindications to the use of Influvac, there are not so many of them. Particular attention should be paid to:
- individual intolerance;
- exacerbations of chronic diseases or other acute processes in the body;
- allergies to any components of the drug.
Children under six months of age are also not vaccinated.
In principle, we are talking about quite typical indications and contraindications for such vaccines.
It is also undesirable to combine several vaccinations within one day, since this increases the likelihood of complications. In case of emergency, vaccines are needed in various limbs.
People are often afraid of being vaccinated because of possible side effects... But it should be said that in this case one has to deal with myths and stereotypes: usually, on the contrary, among those who are not vaccinated, the percentage of deaths from pneumonia (complications of influenza disease) is high.
There is no evidence of death from antiviral vaccinations. Only if there are contraindications, people should not be given such vaccinations - which is why a thorough examination of any patient before vaccination is imperative (especially, this applies to children).
Alas, during mass vaccination in kindergartens, schools and other institutions of this kind, only a superficial examination of the vaccinated children is carried out. Accordingly, from time to time you have to deal with unpleasant cases.
Even the likelihood of anaphylactic shock after an injection is one in a million.
Side effects
You need to know that sometimes (quite rarely, but there are) the Influvac vaccine may have some side effects.
Usually you have to deal with:
- redness at the vaccination site, the appearance of puffiness, soreness and dense infiltration;
- headaches;
- sweating;
- joint and muscle pain;
- fever and chills;
- increased fatigue;
- swollen lymph nodes;
- allergic reactions (skin rashes and even anaphylactic shock);
- convulsions;
- vasculitis.
As statistics show, often side effects associated with local reactions, but the likelihood of their occurrence is only 4 percent (and then, if they happen, it is traditionally a question of redness). Probability common manifestations- like a headache and a boost temperature indicators- equal to 1 percent.

Sometimes headache after vaccination
Severe reactions after the use of Influvac were not observed. Even if the fever begins, it lasts for a maximum of 1 day. Preservation of the reddened skin area is possible for a couple of days. All these manifestations do not require any treatment procedures and pass by themselves.
Perhaps the most dangerous side effect observed after vaccination is anaphylactic reaction. That is why an anti-shock first aid kit with epinephrine or adrenaline should always be in the doctor's office.
Judging by the reviews about the influenza vaccine Influvac, in most cases people do not have to deal with unpleasant consequences after vaccination. But this vaccination solves its immediate task, that is, it strengthens the immune system and significantly reduces the risks of infection during the epidemic.
A few more nuances
If soon after vaccination it is necessary to be tested for hepatitis C or tested for HIV, it is advisable to wait at least a few days. If this is not done, serologic testing will show false positive results.
How compatible is Influvac with alcohol?? In principle, such compatibility has not been studied by physicians, but it is better to drink any alcohol-containing drinks for some time after vaccination and to refuse immediately before it.
You can also talk about a weak specific immune response in the elderly, children and individuals who receive immunosuppressive therapy.
Children under six months of age are not vaccinated.
Reactions to vaccination in representatives younger age for vaccination are observed more often than in adults. Along with the typical increase in temperature indicators, cough and runny nose (stuffy nose) may appear.
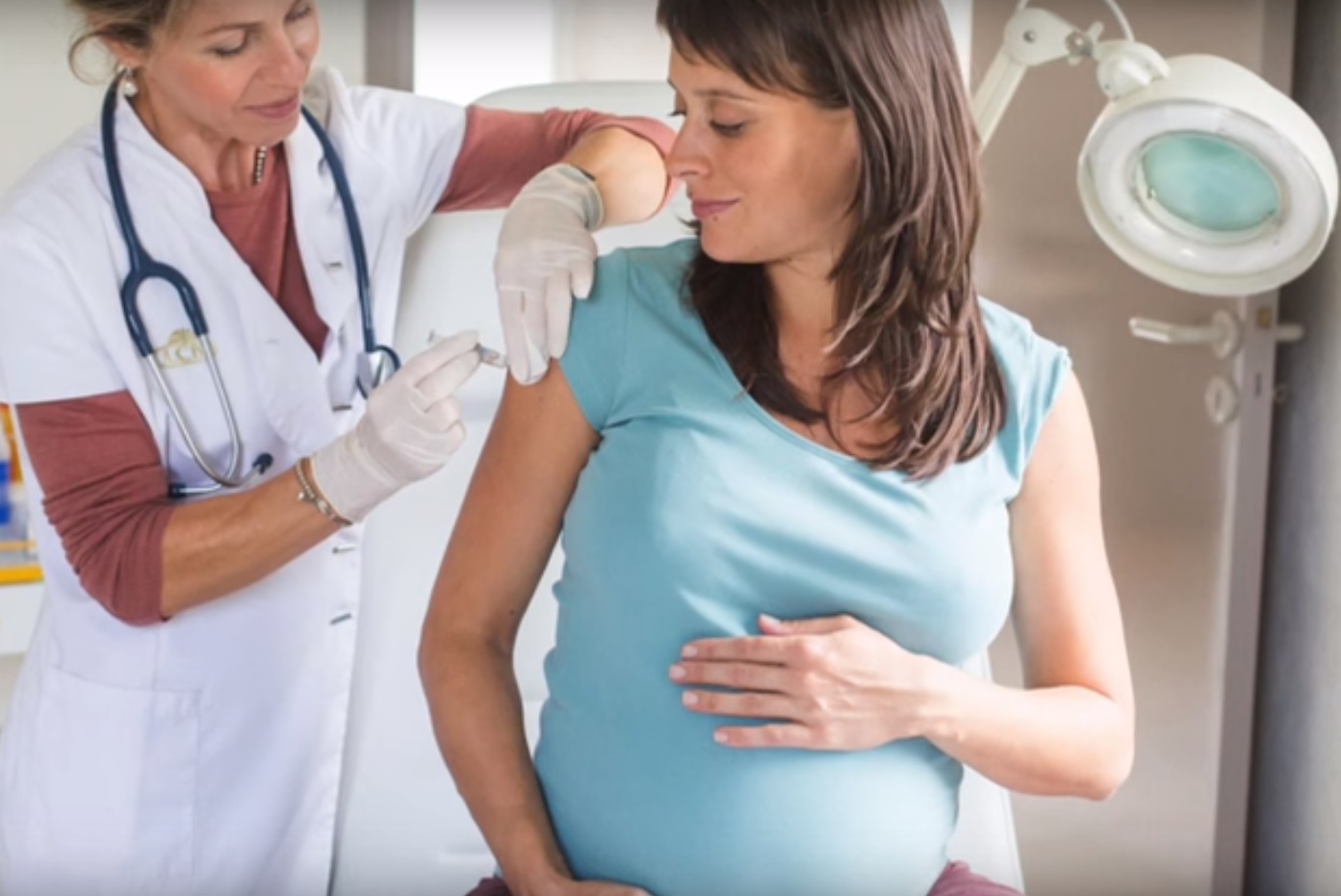
Pregnant women are vaccinated from the second trimester
During pregnancy, this vaccine is allowed in the second trimester. Since the vaccine is clean, it will not cause any harm to the fetus. Women at risk and prone to influenza infection are vaccinated, regardless of how long they are pregnant.
Breastfeeding mothers are also allowed to be vaccinated.
Vaccination action
The flu shot Influvac works as follows:
- Surface antigens enter the human body.
- For a couple of weeks, the accumulation of specific antibodies occurs.
- When the influenza virus gets in, the antibodies begin to destroy its shell and destroy it.
By the way, the effectiveness of the described vaccine has been tested in special clinical studies. According to their results, Influvac provides protection against viral infection in 90 percent of cases what says about him high rates immunogenicity.
Influvac significantly reduces the likelihood of infection
According to static data, after vaccination, people are less likely to get sick not only with the flu, but also with other acute respiratory infections. The explanation for this is the presence of the same surface genes in respiratory viruses as in influenza infections.
It takes two weeks to form immunity... Its duration is almost the entire year. This is why people are vaccinated most often in the first months of the fall.
How is Influvac administered?
Release of this drug carried out directly in syringes, suggesting single use... This eliminates the need to pick up and dissolve the medicine. One dose contains 0.5 milliliters.
- A single administration of one dose is prescribed for children, starting with three years of age, and adults.
- Until the age of three, children are given half the dose.
- A child who has not yet been vaccinated is vaccinated twice: 0.25 milliliters and another 0.25 milliliters a month later.
The introduction is made into the muscles or under the skin - in the thigh or shoulder. As a rule, this vaccination is not done in the buttock, since this place is characterized by a large thickness of subcutaneous fat.
In no case is Influvac administered intravenously!
Before vaccinating a patient, the doctor visually checks whether there are any suspensions and foreign particles in the vaccine. He pays special attention to the shelf life of the drug used, as well as the conditions of its storage.
Vaccinated in the shoulder or thigh
People sometimes ask - is it allowed to wash after having received the Influvac vaccine? It is not recommended to wet the area of the skin where the injection was made throughout the day.
Vaccine analogues
Perhaps the only drawback of the described drug, people call its cost. Indeed, it is more expensive than many domestic vaccines, and Influvac is usually not used for free mass vaccination. Such vaccination can be ordered individually, which many people do on the eve of the epidemic.
Of the analogs of this drug, that is, other subunit and split vaccines, they are most often used:
- the Italian Agrippal;
- domestic "Grippol" or "Grippol Plus";
- the Belgian "Fluarix";
- French "Vaxigrip";
- German "Begrivak".
Each person can choose their own vaccination for themselves, having carefully familiarized themselves with its characteristics, indications and contraindications.
Influvac vaccine has analogues
Influvac does not contain any harmful impurities(during manufacture it undergoes high-quality cleaning) and is completely harmless to health. It is not used only if there are contraindications. And from the side effects, people who are vaccinated often face headaches and redness.
What conclusion can be drawn?
To be sure of the effectiveness and safety of the Influvac flu shot, you can read the reviews about this drug. In most cases, people confirm that they are less likely to get sick after they have been given this vaccine - not only do they not get the flu, but they also avoid any other acute respiratory infections (although, of course, in this case, there are no special guarantees).
Sometimes, even after vaccination, a person "picks up" the infection, but the disease can still be dealt with faster and dangerous complications avoided.
Last updated description by manufacturer 08.08.2007
Filterable list
ATX
Pharmacological group
3D images
Composition and form of release
| Suspension for intramuscular and subcutaneous administration | 1 dose (0.5 ml) |
| One dose of the vaccine (0.5 ml) contains hemagglutinin (HA) and neuraminidase (HA) of the following viral strains: | |
| A (H 3 N 2) | 15 μg HA |
| A (H 1 N 1) | 15 μg HA |
| B | 15 μg HA |
| Excipients: potassium chloride; potassium dihydrogen phosphate; sodium phosphate dihydrate; sodium chloride; calcium chloride dihydrate; magnesium chloride hexahydrate; water for injections |
in disposable syringes complete with 0.5 ml injection needles; in a pack of cardboard 1 or 10 sets.
Description of the dosage form
Transparent colorless liquid.
Characteristic
Influenza vaccine, subunit, inactivated... Influvac ® is a trivalent inactivated influenza vaccine consisting of surface antigens (HA, HA) of influenza A and B viruses grown on chicken embryos. Antigenic composition influenza vaccine updated annually according to WHO recommendations.
pharmachologic effect
pharmachologic effect- formative development of specific immunity.Pharmacodynamics
Forms specific immunity to influenza viruses of types A and B, which occurs, as a rule, 14 days after vaccination and lasts up to 1 year.
Indications for Influvac ® (subunit inactivated influenza vaccine)
Prevention of influenza in adults and children from 6 months.
persons over 65 years old, regardless of their state of health;
patients with respiratory diseases;
patients with cardiovascular diseases of any etiology;
patients with chronic renal failure;
patients with diabetes mellitus;
patients with immunodeficiency diseases (HIV infection, malignant blood diseases, etc.) and patients receiving immunosuppressants, cytostatics, radiation therapy or high doses of corticosteroids;
children and adolescents (from 6 months to 18 years old) receiving medications containing acetylsalicylic acid, and therefore subject to increased risk the development of Reye's syndrome due to influenza infection;
pregnant women (II-III trimester). Pregnant women in high-risk categories should be vaccinated regardless of the stage of pregnancy.
Contraindications
hypersensitivity to chicken protein or to any other component of the vaccine;
strong temperature or allergic reactions after previous vaccination with subunit influenza vaccines.
Vaccination is postponed until the end of acute manifestations of the disease and exacerbation chronic diseases... For mild ARVI, acute intestinal and other diseases, vaccination is carried out immediately after the temperature has returned to normal.
Application during pregnancy and lactation
Application experience shows that Influvac ® has no teratogenic or toxic effect on the fetus. Influvac ® vaccine can be used during lactation.
Side effects
From the circulatory and lymphatic system: rarely, thrombocytopenia.
From the side immune system: rarely - allergic reactions are possible; very rarely - anaphylactic shock.
From the side nervous system: often - headache; rarely - paresthesia, convulsions, encephalomyelitis, neuritis, Guillain-Barré syndrome.
However, no convincing data have been established on the association of these reactions with vaccination.
From the side vascular system: very rare - vasculitis with transient violation kidney function.
General disorders: often - fatigue and neuralgia, which do not require treatment and disappear in 1-2 days.
Local reactions: redness, swelling, soreness, induration, ecchymosis.
Systemic reactions: fever, malaise, trembling, sweating, soreness in muscles and joints.
In the room where vaccination is carried out, it is necessary to have drugs for the treatment of anaphylactic shock (adrenaline, glucocorticoids, etc.).
Interaction
Influvac ® can be used simultaneously with other vaccines (vaccines should be injected into different parts of the body with different syringes). Increased side effects are possible.
If the patient is receiving immunosuppressive therapy, the immune response may be reduced.
After vaccination, it is possible to receive false positives serological tests (when conducting an enzyme-linked immunosorbent assay (ELISA), which are due to the production of IgM after vaccination.
Incompatibility: cases of incompatibility are unknown.
Method of administration and dosage
V / m or PC(deep). It is strictly forbidden to inject the drug intravenously. Immunization is carried out annually in autumn period... Adults and adolescents (from 14 years old) - 0.5 ml once, children: from 6 months to 3 years old - 0.25 ml, from 3 to 14 years old - 0.5 ml once; children who have not previously had the flu and have not been vaccinated, as well as patients with immunodeficiency - twice with an interval of 4 weeks.
Precautionary measures
The drug may contain an undetectable residual amount of gentamicin, therefore, when vaccinating persons with increased sensitivity caution should be exercised with regard to aminoglycosides.
special instructions
The vaccine retains its properties for 12 months. The expiration date is considered to be June 30 of the year following the year of issue. Do not use after the expiration date stated on the package.
Directions for handling disposable syringes
Before being administered, the vaccine must warm up to room temperature... Immediately before injection, the syringe must be shaken, remove the protective cap from the needle and remove air from the syringe, holding it in an upright position with the needle up and slowly pressing the plunger. With the introduction of a dose of 0.25 ml, the movement of the syringe plunger is stopped at the moment when it inner surface reaches the bottom of the needle holder.
Manufacturer
Solvay Pharmaceuticals B.V., The Netherlands.
Storage conditions of the drug Influvac ® (subunit inactivated influenza vaccine)
In a dark place at a temperature of 2-8 ° C (do not freeze).Keep out of the reach of children.
Shelf life of Influvac ® (subunit inactivated influenza vaccine)
12 monthsDo not use after the expiration date printed on the package.
In September-October, we recommend that you protect yourself and your children from influenza with modern vaccines.
It is important to vaccinate before the start of the season colds and flu, because it takes about a month to form a full-fledged immune response.
Last year, during a flu epidemic, many wanted to get vaccinated, and the vaccine was no longer available.
Don't repeat the mistakes! Get your flu vaccine on time!
The center uses flu vaccine Influvac (Influvac, manufactured by Abbott, Netherlands)... Influvac vaccine is specially formulated for season 2016/2017 according to the recommendations The World Organization health care for northern hemisphere and a European Union (EU) decision.
The influenza vaccine Influvac is certified for use in Russia.
In accordance with the instructions for use, when vaccinating against influenza, the Influvac vaccine is administered intramuscularly or deeply subcutaneously:
- adults and adolescents (children over 14 years old) - in a dose of 0.5 ml once;
- children from 3 to 14 years old - in a dose of 0.5 ml once;
- children from 6 months to 3 years old - in a dose of 0.25 ml once.
For children who have not previously been vaccinated against influenza, it is recommended that the vaccine be given twice with an interval of 4 weeks.
Influvac vaccines form the development of specific immunity in influenza viruses of types A and B, which is created, as a rule, 14 days after vaccination and lasts up to 1 year.
Flu shots are performed by a board-certified pediatric immunologist.
Vaccines are transported and stored in strict accordance with the cold chain.
Influvac ® (Influvac ®)
Influenza vaccine, subunit, inactivated.
Registration certificate:
in Russia: P No. 015694/01
in Kazakhstan: RK-BP-5-№000287
Dosage form:
Suspension for intramuscular and subcutaneous administration.
Influvac® is a trivalent inactivated influenza vaccine consisting of surface antigens (hemagglutinin (HA), neuraminidase (HA)) of influenza A and B viruses grown in chicken embryos. The antigenic composition of the influenza vaccine is updated annually in accordance with the recommendations of the World Health Organization.
One dose of the vaccine (0.5 ml) contains HA and HA of the following viral strains:
* after the name of the strain, the name of the type recommended by WHO for the current epidemic season of influenza is taken out.
Excipients: potassium chloride, potassium dihydrogen phosphate, sodium phosphate dihydrate, sodium chloride, calcium chloride dihydrate, magnesium chloride hexahydrate, water for injection.
DescriptionTransparent colorless liquid.
Immunological propertiesThe vaccine forms the development of specific immunity to influenza viruses of types A and B, which occurs, as a rule, 14 days after vaccination and lasts up to 1 year.
AppointmentPrevention of influenza in adults and children from 6 months.
- persons over 65 years old, regardless of their state of health.
- patients with respiratory diseases;
- patients with cardiovascular diseases of any etiology;
- patients with chronic renal failure;
- patients with diabetes mellitus;
- patients with immunodeficiency diseases (HIV infection, malignant blood diseases, etc.) and patients receiving immunosuppressants, cytostatics, radiation therapy or high doses of corticosteroids;
- children and adolescents (from 6 months to 18 years) who have been receiving medications containing acetylsalicylic acid for a long time and, therefore, are at an increased risk of developing Reye's syndrome due to influenza infection.
- pregnant women in the 2nd and 3rd trimesters of pregnancy. Pregnant women in high-risk categories should be vaccinated regardless of the stage of pregnancy.
Hypersensitivity to chicken protein or any other component of the vaccine, severe temperature or allergic reactions after previous vaccination with subunit influenza vaccines. Vaccination is postponed until the end of acute manifestations of the disease and exacerbation of chronic diseases. For mild ARVI, acute intestinal diseases and other vaccinations are carried out immediately after the temperature has returned to normal.
Method of administration and dosage- Dose for adults and adolescents (from 14 years old): 0.5 ml. The vaccine is given once.
- Dose for children from 6 months of age to 3 years: 0.25 ml; dose for children from 3 to 14 years old: 0.5 ml. The vaccine is given once. Children who have not previously had the flu and have not previously been vaccinated are recommended to inject the vaccine twice with an interval of 4 weeks.
- Patients with immunodeficiency are recommended to inject the vaccine twice with an interval of 4 weeks.
Mode of application. Immunization is carried out annually in the fall. The vaccine is administered intramuscularly or deeply subcutaneously. It is strictly forbidden to administer the drug intravenously.
Interaction with other medicinal products and other forms of interactionInfluvac® can be used concurrently with other vaccines. In this case, vaccines should be injected into different parts of the body with different syringes. Increased side effects are possible. If the patient is receiving immunosuppressive therapy, the immune response may be reduced.
After vaccination, it is possible to receive false positives serological tests, with enzyme-linked immunosorbent assay (ELISA), which are due to the production of IgM after vaccination.
Application during pregnancy and lactationExperience has shown that Influvac® does not have a teratogenic or toxic effect on the fetus. Influvac® vaccine can be used during lactation.
Side effectsOn the part of the circulatory and lymphatic system: Rarely: thrombocytopenia
From the immune system: In rare cases, allergic reactions are possible, in very rare cases - anaphylactic shock.
From the nervous system: Often: headache. Rarely: paresthesias, convulsions, encephalomyelitis, neuritis, Guillain-Barré syndrome. However, no convincing data have been established on the association of these reactions with vaccination.
From the vascular system: Very rare: vasculitis with transient renal impairment
General disorders: Often: fatigue, neuralgia, not requiring treatment and disappearing in 1-2 days. Local reactions: redness, swelling, soreness, induration, ecchymosis. Systemic reactions: fever, malaise, tremors, sweating, soreness in muscles and joints.
In the room where vaccination is carried out, you must have medicines for the treatment of anaphylactic shock (adrenaline, glucocorticoids, etc.)
special instructionsThe drug may contain an undetectable residual amount of gentamicin, therefore, caution should be exercised when vaccinating individuals with hypersensitivity to aminoglycosides.
Influence on the ability to drive a car or control machines and mechanismsInfluvac® does not affect the ability to drive or use machines and mechanisms.
IncompatibilityNo incompatibilities are known.
Package0.5 ml in a 1.0 ml disposable syringe with a needle closed with a plastic cap. 1 or 10 syringes in a cardboard holder or in a sealed plastic holder. The cardboard holder is placed in a cardboard box with first opening control. Sealed plastic holder - in a cardboard box. Instructions for use are included in a cardboard box.
Shelf life12 months. In this case, the expiration date is considered to be June 30 of the year following the year of issue. Do not use after the expiration date indicated on the package.
Storage conditionsIn accordance with SP 3.3.2.1248-03. Store in a dark place at a temperature of 2 ° C to 8 ° C, do not freeze. Keep out of the reach of children.
Vacation conditionsConditions containing 1 syringe are dispensed from pharmacies on prescription.
Conditions containing 10 syringes are released by medical and prophylactic institutions.
Directions for handling disposable syringesThe vaccine must warm to room temperature before being administered. Shake the syringe just before injection. Remove the protective cap from the needle and remove air from the syringe by holding it in an upright position with the needle up and slowly pushing the plunger. With the introduction of a dose of 0.25 ml, the movement of the syringe plunger is stopped at the moment when its inner surface reaches the lower edge of the needle retainer.
