Influvac and influenza plus comparison. Influvac ® (subunit inactivated influenza vaccine) (Influvac ®)
The main danger posed by a disease such as influenza is the occurrence severe consequences... There are many different drugs available to treat the flu to help speed up the healing process. In addition to medications to treat the flu, there are also medications that can help prevent the onset of the disease. These are special influenza vaccines, which are produced not only abroad, but also in Russia. Every year the influenza virus mutates, so the use of a vaccine with the same composition is irrational. Influvac is used to vaccinate the population against influenza. What is included in this vaccine, as well as whether it is allowed to be used for children, we will find out further.
Features of vaccination "Influvac"
The Influvac vaccine is a drug that basically contains inactive influenza viruses that are artificially introduced into the human body. Initially, influenza viruses undergo a cultivation process in chicken embryos, after which they are selected and neutralized using formaldehyde. Through special technology influenza antigens are selected, after which the Influvac vaccine is created. The viruses contained in the vaccine are not capable of provoking an illness, but are intended for the human body to develop the appropriate immunity.
The influenza vaccine Influvac is produced in the Netherlands by the pharmaceutical company Abbott Biologicals BV. Since 1988 given view vaccines began to be used in Russian Federation... The main components of the Influvac vaccine are antigens in the purified form of influenza A and B viruses. The composition of the vaccine changes annually, otherwise the effectiveness of the drug would be zero. Depending on which influenza virus is expected, the appropriate strains are added to the vaccine.
It's important to know! When the injection is released, the packaging indicates the season for which the drug is intended (for example, 2016-2017).
Vaccination is recommended every year, and for this, vaccines appropriate for the season should be used. The vaccine contains particles of viruses such as H3N2, H1N1 and B. In addition, additional components are included in the vaccine:
- formaldehyde;
- polysorbate;
- sodium citrate;
- chicken protein;
- sodium and potassium chlorides.
It's important to know! If a person has signs of intolerance to these components, then vaccination with just such a drug should be excluded.
Influvac vaccine is well tolerated not only by adults, but also by children. Influvac instructions provide for the use of the drug for children from the age of six months. People of the following categories must be vaccinated against influenza:
- People who have frequent illnesses ARI.
- Patients over the age of 60-65 years.
- Patients with the disease diabetes mellitus, as well as with immunodeficiency.
- If you have heart disease and vascular system.
- During pregnancy from the second trimester.

It's important to know! Not only the above-mentioned persons can be vaccinated, but also all citizens, starting from 6 months. Vaccination is optional, so it is optional.
Influvac injection action
When a vaccine against influenza "Influvac" is administered, the maximum protection of immunity is not formed immediately, but after 2 weeks. When surface antigens enter the body, an immune response occurs. In the case when influenza viruses enter the body that has been vaccinated, the antibodies destroy the envelope, and also provoke the death of the viral envelopes.
It's important to know! Studies on the effectiveness of Influvac injection have shown that the drug is effective in 90% of cases.
Based on the research, it was concluded that Influvac has not only increased immunogenicity, but also high degree protection from illness. It was also concluded that the injection has a positive effect not only on the flu, but also on the signs of acute respiratory infections. Vaccination is best done in early autumn, even before the onset of cold weather, and its effectiveness lasts about a year. Doctors recommend repeating the vaccination every year before the beginning of autumn or with the arrival of spring, when the immune system is also weakened after winter.
Features of the injection
The drug for the prevention of influenza and influenza diseases is available in disposable syringes, and one dose in a syringe is designed exclusively for one patient. It is not necessary to collect or dissolve anything for vaccination, since the drug is already ready for use. One dose of the drug contains 0.5 ml of the drug. For adults, children and adolescents from 3 years old, the drug is used in the amount of one dose. Children from 6 months to 3 years old are injected with half the dosage available in the syringe. If a child is vaccinated for the first time, then the vaccination is carried out twice: the first time in the amount of 0.25 or 0.5 ml, and the second time a month later in the appropriate dose.
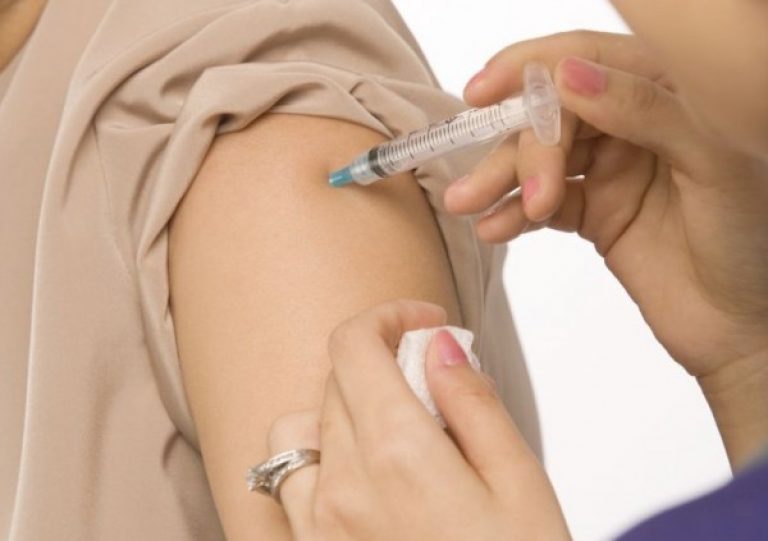
It is better to inject it into the muscle, but it can also be injected under the skin, with the exception of the vein. Health professionals inject the drug into the shoulder or thigh area. It is not recommended to inject medication into the buttock, since a large number of adipose tissue will lead to slow absorption of the drug.
It's important to know! Before injecting into the body, medical worker is obliged to make sure that the drug has not expired.
Before using the injection, it is recommended to warm it up to room temperature, which will speed up the process of assimilation by the body. After vaccination, it is not recommended to wet the injection site of the vaccine; it is especially contraindicated to get dirty substances at the injection site.
In what situations is Influvac contraindicated for use?
In the instructions for the use of Influvac, a number of contraindications are listed, in the presence of which it is strictly forbidden to vaccinate. These contraindications include:
- the presence of signs of individual intolerance;
- in the presence of acute respiratory diseases: ARVI and other ailments that contribute to an increase in body temperature;
- children under 6 months of age.
This number of contraindications is minimal, which indicates the safety of the drug. If the patient has signs of the development of the disease, it is recommended to wait until the body temperature returns to normal, and then resort to vaccination. It is not recommended to vaccinate the patient with several injections on the same day. This can cause complications, as well as lead to the development of side symptoms.
It's important to know! If there is a need to use several vaccines on one day, then it is desirable to inject them into different limbs.
Adverse symptoms when using Influvac injection
Despite the safety of Influvac injection, the development of side symptoms is possible, manifested in the following form:
- the occurrence of headaches;
- redness, swelling, swelling, and soreness of the injection site;
- chills and fever;
- weakness and fatigue;
- vasculitis;
- swollen lymph nodes;
- muscle pain.
On the basis of statistics, it was found that if side symptoms appear, they are expressed in the form of local reactions. For the early elimination of puffiness and soreness at the injection site, it is recommended to make an iodine mesh. The incidence of such complications is 4%. Headaches and chills occur in the amount of 1%. Severe reactions of the body to the use of the vaccine were not detected, but if any occur, it is required to immediately inform the doctor. Usually, side symptoms disappear the next day, and in exceptional cases - on the second day.
One of the most dangerous reactions to the vaccine is allergy, which manifests itself in the form of anaphylactic shock. In the event of allergic reactions, and especially the development of anaphylactic shock, it is required immediate hospitalization a patient with the need to administer epinephrine or epinephrine.
It is not recommended to vaccinate a patient who is in a condition alcoholic intoxication... Despite the fact that the reaction of the vaccine to alcohol has not been studied, the development of serious complications is not excluded.
Vaccination of children and women during pregnancy
For children, an influenza injection such as Influvac is approved for use from the age of six months. It is important to note that the injection should be administered even before the onset of influenza, since this prophylactic drug, the main purpose of which is to exclude the occurrence of influenza. Such a vaccine cannot cure influenza, therefore, if a person is ill, then it is necessary to be treated, and after recovery, you can get vaccinated.
It's important to know! There are times when a patient was vaccinated, and after a while he fell ill with the flu. This means that the influenza virus entered the body even before it had time to develop immunity (this requires 14 days after the injection), or the person did not get sick with the flu, but with ARVI or ARI. For clarification, you need to contact your doctor. If the doctor confirms the diagnosis of influenza, then it is necessary to find out which vaccine against the disease was administered.
Children are more likely to react to the vaccine than adults. It is possible that the symptoms of injection in children will be presented in the form of a cough and a runny nose. These symptoms can last from a few days to 2 weeks.
Instruction for the Influvac vaccine provides for the use of the drug for pregnant women, but starting from the second trimester. The cleansing of the injection is so great that the drug has no effect negative impact on the fetus. It is especially important to use the injection for women during pregnancy who are at risk of getting the flu. It is allowed to vaccinate not only pregnant women, but also nursing mothers.
Are there analogues of Influvac
A significant drawback that Influvac injection has is the high cost of the drug. Unlike the Sovigripp injection, which is purchased by the state and is free of charge for the vaccination of the population, the state does not allocate funds for the Influvac vaccine, so anyone who buys the drug can get vaccinated.
The following drugs are analogous to Influvac injection:
- Vaxigripp, which is a split vaccine manufactured in France.
- Grippol manufactured in Russia.
- Agrippal made in Italy.
- Fluarix, which is a split vaccine containing not only antigens, but also virus molecules. Manufactured in Belgium.
- Begrivak. German analogue of the vaccine Influvac.
Which flu vaccine to give preference depends on the person who wants to. All vaccines are roughly the same, but may differ only in price.
In conclusion, it should be noted that vaccination is recommended for all segments of the population in order to avoid the occurrence of an exacerbation of outbreaks of the virus. Influvac injection is not only one of the most effective, but also safe, due to the possibility of using it for children from 6 months. After vaccination, patients receive increased immunity to strains of the influenza virus, but maximum protection occurs only after 14 days.
The effectiveness of the vaccine is 90%, which is due to the quality of the drug. After vaccination, adverse symptoms may occur, which is normal occurrence organism to such a drug. Usually, side symptoms disappear on day 2, but if complications arise, you need to go to the hospital again.
Influvac- influenza subunit inactivated vaccine for the prevention of influenza in adults and children from 6 months of age. Develops specific immunity to influenza A and B viruses, provides protection against influenza. Possesses high immunogenicity and low reactogenicity. Vaccinations can be given to anyone who wants to reduce the risk of getting the flu and avoid possible complications flu.
Latin name:
INFLUVAC / INFLUVAC.
Composition and form of release:
Influvac suspension for injection (for intramuscular and subcutaneous administration) in disposable syringes "Dufarject", 0.5 ml, 1 or 10 pcs. in a package, complete with injection needles.
1 dose (0.5 ml) of the Influvac vaccine contains: hemagglutinin (15 μg HA each) and neuraminidase of three influenza viruses. The antigenic composition of the influenza vaccine Influvac is updated annually according to the recommendations The World Organization health care; each year, the viral strains for the new season are indicated.
Properties / Action:
Influvac is a subunit trivalent inactivated vaccine for the prevention of 3rd generation influenza.
Influvac contains purified surface antigens of influenza A and B viruses - hemagglutinin (HA) and neuraminidase (HA), which are obtained from certain influenza vaccine strains grown in chicken embryos. The antigenic composition of the influenza vaccine Influvac is updated annually in accordance with the recommendations of the World Health Organization (WHO).
Influvac vaccine develops specific immunity to influenza A and B viruses and provides protection against influenza. The purified antigens (hemagglutinin and neuraminidase) contained in Influvac vaccine cause an increase in the antibody titer required for protective effect, which usually occurs 14 days after injection. The duration of post-vaccination immunity lasts up to 12 months. The degree of protection in the elderly and children younger age lower than in adults.
High immunogenicity and very low reactogenicity, due to the high-tech production of the Influvac vaccine, makes it possible to prevent influenza in adults and children from 6 months of age.
Pharmacokinetics:
Influvac - immunobiological drug; pharmacokinetic studies have not been conducted.
Indications:
Influvac is indicated for the prevention of influenza in adults and children from 6 months of age.
Vaccinations can be given to anyone who wants to reduce the risk of getting the flu and to avoid possible complications.
Vital essential Influvac has for patients at risk:
- patients with respiratory diseases;
- patients with cardiovascular diseases of any etiology;
- patients with chronic renal failure;
- sick diabetes mellitus;
- patients with staphylococcal infections;
- patients with immunodeficiencies (HIV infection, malignant blood diseases, etc.);
- patients receiving immunosuppressants, cytostatics, high doses of corticosteroids, radiation therapy;
- persons over 65 years old, regardless of their state of health;
- children and adolescents (from 6 months to 18 years old) who have been receiving drugs containing acetylsalicylic acid, and therefore subject to increased risk the development of Reye's syndrome (Reye) due to influenza infection;
- pregnant women in the 2nd and 3rd trimesters of pregnancy.
Method of administration and dosage:
Immunization is carried out annually in autumn period... Influvac is administered intramuscularly or deeply subcutaneously. It is strictly forbidden to administer the drug intravenously.
Influvac dose for adults and adolescents over 14 years of age: 0.5 ml once.
Influvac dose for children from 6 months of age to 3 years: 0.25 ml once.
Influvac dose for children from 3 to 14 years old: 0.5 ml once.
Children who have not previously had the flu and have not previously been vaccinated are advised to administer two doses of Influvac with an interval of 4 weeks.
Patients with immunodeficiency are recommended to administer two doses of Influvac with an interval of 4 weeks.
Dufarject.
The design of the Dufarject syringe is based on a unique original device. The syringe is easy to handle and provides a completely safe and painless injection of the exact dose of Influvac vaccine.
Directions for handling disposable syringes.
The vaccine must be warmed to room temperature prior to administration. Shake the syringe immediately before injection. Remove the protective cap from the needle and remove the air from the syringe by holding it in an upright position with the needle up and slowly pushing the plunger. With the introduction of a dose of 0.25 ml, the movement of the syringe plunger is stopped at the moment when it inner surface reaches the bottom of the needle holder.
Overdose:
There are no data on an overdose of Influvac vaccine.
Contraindications:
- individual intolerance (including history of hypersensitivity) chicken protein or other components of Influvac;
- strong fever or severe allergic reactions after a previous vaccination with subunit influenza vaccines;
- acute diseases and exacerbations chronic diseases(vaccination is postponed until the end of acute manifestations).
In case of mild ARVI, acute intestinal and other diseases, vaccination with Influvac is carried out immediately after the normalization of body temperature.
Application during pregnancy and lactation:
Before vaccination during pregnancy, the risk of possible side effects with the risk of getting the flu and the risk of developing complications from the flu. Experience has shown that Influvac can be used without any concerns about the fetus during pregnancy; Influvac has no teratogenic or toxic effect on the fetus in pregnant women.
Influvac can be used during lactation (breastfeeding).
Side effect:
The safety of using the Influvac vaccine:
Since Solway started producing Influvac, only a few cases of side effects have been reported in the course of research. The fact that over 23 million doses of Influvac vaccine have been administered over a five-year follow-up period and no known side effects have been observed that could be associated with its action serves as an additional guarantee for the safety of the vaccine.
The ratio inherent to Influvac vaccine possible risk and the expected beneficial effect is highly appreciated in the context of the prevention of influenza and its complications.
Possible side effects of Influvac vaccine:
Local side effects such as redness, swelling and soreness at the injection site are possible. In very rare cases, there may be an increase in regional lymph nodes.
Systemic side effects rarely occur: feeling tired, headache, sweating, fever, soreness in muscles and joints. These symptoms go away on their own in 1-2 days.
In very rare cases, the appearance of neuralgia, parasthesia, seizures, and short thrombocytopenia is possible.
In even rarer cases, development of neurological disorders, vasculitis with temporary renal dysfunction.
In extremely rare cases, allergic reactions are accompanied by the development of shock.
Special instructions and precautions:
IT IS STRICTLY FORBIDDEN TO INJECT THE PREPARATION INTRAVENOUSLY!
Vaccination with Influvac is recommended primarily for patients with respiratory diseases, cardiovascular diseases, chronic renal failure, diabetes mellitus, staphylococcal infections, immunodeficiency diseases; patients receiving immunosuppressants, cytostatics, radiation therapy or high-dose GCS. Vaccination is especially recommended for children and adolescents aged 6 months to 18 years who have been receiving drugs containing acetylsalicylic acid for a long time and, therefore, are at increased risk of developing Reye's syndrome due to influenza infection.
Influvac vaccination is postponed until the end of acute manifestations of the disease and exacerbation of chronic diseases. For mild ARVI, acute intestinal diseases vaccination is carried out immediately after the temperature has returned to normal.
Patients with a history of side effects from the administration of various vaccines, if necessary, simultaneous vaccination with Influvac and other vaccines, the drugs should be administered at intervals of at least 3 weeks.
In the room where vaccination is carried out, you must have medicines for the treatment of anaphylactic shock.
After vaccination, it is possible to receive false positives serological tests in enzyme-linked immunosorbent assay (ELISA), which are due to the production of IgM after vaccination.
Vimpact on the ability to drive a car and control machines and mechanisms:
Influvac does not affect the ability to drive and use machines and mechanisms.
Drug interactions:
Cases of incompatibility of Influvac with other drugs are unknown.
Influvac can be used concurrently with other vaccines. In this case, vaccines should be injected into different limbs, with different syringes, keeping in mind the theoretical possibility of increasing side effects. In patients with already known reactions of this kind, separate administration of drugs with an interval of at least 3 weeks is recommended.
If the patient is receiving immunosuppressive therapy, the immune response may be reduced.
Storage conditions:
Store in a dark place at a temperature of 2 ° C to 8 ° C (in the refrigerator). Do not freeze!
Keep out of the reach of children!
Influvac retains its properties for 12 months. In this case, the expiration date expires on June 30 of the year following the year of release (to avoid confusion with vaccines of the old and new composition).
Do not use after the expiration date indicated on the package.
Terms of dispensing from a pharmacy - according to a doctor's prescription.
Influvac ® (Influvac ®)
Influenza vaccine, subunit, inactivated.
Registration certificate:
in Russia: P No. 015694/01
in Kazakhstan: RK-BP-5-№000287
Dosage form:
Suspension for intramuscular and subcutaneous administration.
Influvac® is a trivalent inactivated influenza vaccine consisting of surface antigens (hemagglutinin (HA), neuraminidase (HA)) of influenza A and B viruses grown in chicken embryos. The antigenic composition of the influenza vaccine is updated annually in accordance with the recommendations of the World Health Organization.
One dose of the vaccine (0.5 ml) contains HA and HA of the following viral strains:
* after the name of the strain, the name of the type recommended by WHO for the current epidemic season of influenza is taken out.
Excipients: potassium chloride, potassium dihydrogen phosphate, sodium phosphate dihydrate, sodium chloride, calcium chloride dihydrate, magnesium chloride hexahydrate, water for injection.
DescriptionTransparent colorless liquid.
Immunological propertiesThe vaccine forms the development of specific immunity to influenza viruses of types A and B, which occurs, as a rule, 14 days after vaccination and lasts up to 1 year.
AppointmentPrevention of influenza in adults and children from 6 months.
- persons over 65 years old, regardless of their state of health.
- patients with respiratory diseases;
- patients with cardiovascular diseases of any etiology;
- patients with chronic renal failure;
- patients with diabetes mellitus;
- patients with immunodeficiency diseases (HIV infection, malignant blood diseases, etc.) and patients receiving immunosuppressants, cytostatics, radiation therapy or high doses of corticosteroids;
- children and adolescents (from 6 months to 18 years) who have been receiving medications containing acetylsalicylic acid for a long time, and, therefore, are at an increased risk of developing Reye's syndrome due to influenza infection.
- pregnant women in the 2nd and 3rd trimesters of pregnancy. Pregnant women in high-risk categories should be vaccinated regardless of the stage of pregnancy.
Hypersensitivity to chicken protein or any other component of the vaccine, severe fever or allergic reactions after previous vaccination with subunit influenza vaccines. Vaccination is postponed until the end of acute manifestations of the disease and exacerbation of chronic diseases. For mild ARVI, acute intestinal diseases, etc., vaccination is carried out immediately after the temperature has returned to normal.
Method of administration and dosage- Dose for adults and adolescents (from 14 years old): 0.5 ml. The vaccine is given once.
- Dose for children from 6 months of age to 3 years: 0.25 ml; dose for children from 3 to 14 years old: 0.5 ml. The vaccine is given once. Children who have not previously had the flu and have not previously been vaccinated are recommended to inject the vaccine twice with an interval of 4 weeks.
- Patients with immunodeficiency are recommended to inject the vaccine twice with an interval of 4 weeks.
Mode of application. Immunization is carried out annually in the fall. The vaccine is administered intramuscularly or deeply subcutaneously. It is strictly forbidden to administer the drug intravenously.
Interaction with other medicinal products and other forms of interactionInfluvac® can be used concurrently with other vaccines. In this case, vaccines should be injected into different parts of the body with different syringes. Increased side effects are possible. If the patient is receiving immunosuppressive therapy, the immune response may be reduced.
After vaccination, it is possible to obtain false-positive results of serological tests, with an enzyme-linked immunosorbent assay (ELISA), which is due to the production of IgM after vaccination.
Application during pregnancy and lactationExperience has shown that Influvac® does not have a teratogenic or toxic effect on the fetus. Influvac® vaccine can be used during lactation.
Side effectsFrom the circulatory and lymphatic system: Rare: thrombocytopenia
From the side immune system: In rare cases, allergic reactions are possible, in very rare cases - anaphylactic shock.
From the side nervous system: Often: headache. Rarely: paresthesia, convulsions, encephalomyelitis, neuritis, Guillain-Barré syndrome. However, no convincing data have been established on the association of these reactions with vaccination.
On the part of the vascular system: Very rare: vasculitis with transient violation kidney function
General disorders: Often: fatigue, neuralgia, not requiring treatment and disappearing in 1-2 days. Local reactions: redness, swelling, soreness, induration, ecchymosis. Systemic reactions: fever, malaise, tremors, sweating, soreness in muscles and joints.
In the room where vaccination is carried out, it is necessary to have medicines for the treatment of anaphylactic shock (adrenaline, glucocorticoids, etc.)
special instructionsThe drug may contain an undetectable residual amount of gentamicin, therefore, when vaccinating persons with increased sensitivity caution should be exercised towards aminoglycosides.
Influence on the ability to drive a car or control machines and mechanismsInfluvac® does not affect the ability to drive or use machines and mechanisms.
IncompatibilityNo cases of incompatibility are known.
Package0.5 ml each in a 1.0 ml disposable syringe with a needle closed with a plastic cap. 1 or 10 syringes in a cardboard holder or in a sealed plastic holder. The cardboard holder is placed in a cardboard box with first opening control. Sealed plastic holder - in a cardboard box. Instructions for use are included in a cardboard box.
Shelf life12 months. In this case, the expiration date is considered to be June 30 of the year following the year of issue. Do not use after the expiration date indicated on the package.
Storage conditionsIn accordance with SP 3.3.2.1248-03. Store in a dark place at a temperature of 2 ° C to 8 ° C, do not freeze. Keep out of the reach of children.
Vacation conditionsConditions containing 1 syringe are dispensed from pharmacies on prescription.
Conditions containing 10 syringes are released by medical and prophylactic institutions.
Directions for handling disposable syringesThe vaccine must warm to room temperature before being administered. Shake the syringe just before injection. Remove the protective cap from the needle and remove air from the syringe by holding it in an upright position with the needle up and slowly pushing the plunger. With the introduction of a dose of 0.25 ml, the movement of the syringe plunger is stopped at the moment when its inner surface reaches the lower edge of the needle retainer.
Afraid of getting the flu? Then you should think about getting vaccinated. Thanks to this event, a person will preserve his health, will not get the flu or get sick, but in a mild form. Immunization is a must, especially when it comes to our kids. Therefore, today we will consider what constitutes the influenza vaccine "Influvac", as well as "Vaxigripp". These are common drugs used in Russia and Ukraine. They are able to prevent getting the flu. Below we will consider the composition of both vaccines, their dosages. We will also determine which of them will be better than the other.
Composition, release form of the vaccine "Influvac"
This drug is sold as a suspension that is administered subcutaneously or intramuscularly. The Influvac vaccine is sold in syringes, which should be disposed of after the injection. The kit also contains needles.
The composition of this vaccine is as follows:
Hemagglutinin and neuraminidase of viral strains of types: A (H3N2), A (H1N1), B.
Auxiliary elements: sodium phosphate dihydrate, potassium, calcium and magnesium chloride, hydrogen phosphate, water for injection.
Dosage of the vaccine "Influvac"
Adults, as well as children from 3 to 14 years old - 0.5 ml once.
Babies from 6 months to 3 years old - 0.25 ml once.
For children who have never been vaccinated before, it is advisable to inject the drug twice with an interval of 4 weeks.
Immunization should be carried out once a year in the fall. 
Side effects after vaccination with Influvac
The use of this remedy can cause such undesirable reactions:
From the side of the central nervous system: often - headache; rarely - neuritis, convulsions, neuralgia, encephalomyelitis, paresthesia.
From the side of the heart and blood vessels: rarely - vasculitis (immunopathological vascular inflammation).
From the musculoskeletal system: often - arthralgia (joint pain), myalgia (pain in the muscle area).
General disorders: often - fatigue, fever, pain in muscles and joints, malaise, trembling, chills.
Other manifestations: often - heavy sweating; rarely - skin manifestations (itching, urticaria, nonspecific rash).
Local reactions: swelling, induration, soreness, redness.
Composition, release form of the vaccine "Vaxigripp"
This drug is also a suspension for administration under the skin or intramuscularly.
The vaccine "Vaxigripp" is produced in syringes or ampoules.
The composition of the preparation is as follows:
The active elements are hemagglutinin and neuraminidase of such viral strains as A (H3N2), A (H1N1), B.
Auxiliary components - sodium, as well as potassium chloride and dihydrogen phosphate, hydrogen phosphate dihydrate, water for injection. 
Immunization rules and dosage of the drug "Vaxigripp"
This flu vaccine can be given:
Subcutaneously in upper part front side shoulder.
Intramuscularly into the deltoid muscle.
For small children - in the anterolateral region of the thigh.
The dosage of the drug is as follows:
Adults and children from 3 years old - 0.5 ml of the product once.
Babies from six months to 3 years old - 0.25 ml of the drug.
People who have not been vaccinated before, as well as those who have not had the flu at all, should be given this vaccine 2 times at intervals of 4 weeks. That is, one dose should be divided equally.
Patients with immunodeficiency should also inject the drug twice - 0.25 ml each with an interval of 1 month. 
Side effects after vaccination with "Vaxigripp"
This remedy also has negative effects. How to understand which vaccine - "Vaxigripp" or "Influvac" - is better? To do this, you can see a list of the side effects of each drug. So, for "Vaxigripp" it is like this:
Often - headache, malaise, sweating, fatigue, pain in muscles and joints, neuralgia.
Rarely - convulsions, paresthesias, neuritis, encephalomyelitis.
Very rarely - allergic manifestations on the body, vasculitis.
Local reactions - induration, soreness, edema at the injection site.
Vaccine cost
The drug "Vaxigripp", the price of which varies in different pharmacies, can be purchased on average for 400 rubles. A person will have to pay this amount for 1 dose of this drug. I wonder how much then the drug "Influvac" costs? The price of this drug ranges from 520-570 rubles.
So what should you choose?
Today, both vaccines are considered the most widely used flu vaccines for children and adults. Both drugs give the same result. However, parents do not stop terrorizing pharmacists and family doctors, so that they would advise which of the two vaccines - "Vaxigripp" or "Influvac" - will be better. The fact is that both drugs hardly differ from each other. Indications for use, release form and even their composition are similar. But on such a point as side effects, there is a difference. So, the "Influvac" product has much larger list possible negative manifestations, while for the drug "Vaxigripp" this list is much shorter. If we consider the cost of these vaccines, then there is also something to catch on. Influvac is slightly more expensive than its competitor. Therefore, if you choose from these two criteria, then you should make a choice in favor of the means "Vaxigripp". Its price is lower, and there are fewer side effects. But it's still better to find out what people think about these two vaccines, and based on their responses, decide for yourself what to choose. ![]()
The drug "Influvac": reviews
Internet users write about this tool mostly only positive opinions. So, those patients who were vaccinated with this drug note that the injection itself is painless, because the needle in the syringe is very thin. Also, rarely does anyone note that after vaccination with this remedy, problems arose. People, on the contrary, praise the drug "Influvac" for the fact that it almost never causes adverse reactions in organism. Also, women and men choose this particular vaccine, because it is imported, which means that it is better purified than domestic. In addition, the composition of the drug is improved annually, because new strains of influenza appear, so the developed immunity may not work. 
There are, however, and negative responses from people. The first thing that parents pay attention to is that Influvac is sold in a standard dosage. That is, it turns out that syringes are the same for both adults and children. This is very inconvenient, because if you give a vaccine to children, then the excess amount of the drug must be drained. It turns out that it is being spent inefficiently. There are also people who say that after being vaccinated with Influvac, their health has deteriorated. To prevent this from happening, you need to give an injection only when the person is completely healthy. That is, no colds he shouldn't have. And if a person listens to the doctor and follows all his recommendations regarding vaccination, then the drug "Influvac" will receive only positive reviews. As for the cost of this tool, people note that its price is quite adequate, and it suits many.
The drug "Vaxigripp": reviews
This vaccine has received positive feedback from patients. Some people get an injection with this drug completely free of charge, others buy it for own funds... However, both of them note the effectiveness of this vaccine: within a year, people do not get the flu. True, there are exceptions when a person nevertheless picks up this virus, but the disease progresses much easier. People also note that although the drug "Vaxigripp" is not the best of the existing ones, it is affordable. And this is an important factor. After all, often all family members have to vaccinate, and this can hit hard on family budget... Therefore, people choose more cheap remedy- "Vaxigripp". Positive reviews are also written by parents who are satisfied that the drug is sold separately for children, that is, in special syringes of 0.25 mg. And there is no need to pour out excess liquid, since the dosage is accurate. 
Expert opinion
What do immunologists and pediatricians say about these vaccines? Which is better: "Vaxigripp" or "Influvac"? Doctors are unanimous in this regard. They believe that these drugs are approximately the same in their properties and effect. They do not single out any of them specifically. And the fact that the “Influvac” is supposedly cleaner is, according to doctors, an insignificant sign that does not affect the development of immunity, as well as its perception by the body. Therefore, if at work they offer to carry out free vaccination, for example, with Vaxigripp, then it is advisable to agree. Since it is stupid to look for the drug "Influvac" in pharmacies, because these vaccines will be the same in terms of effectiveness. Well, if you do not have such an advantage, then indeed, you can purchase either of these two funds yourself. The main thing is to pay attention to the expiration date of the drug, and also follow all recommendations for proper storage and transportation.
Now you know which of the two means - "Vaxigripp" or "Influvac" - is better. And they realized that in fact there is no difference in them. The nuance is that the first product can be sold in a special small dosage (for children). While some of the Influvac preparation will have to be poured out, since the children need to inject only 0.25 mg, and 0.5 mg is in the syringe. Also, one more point is that the "Vaxigripp" suspension is a little cheaper. Well, doctors do not allocate these funds, considering that they are approximately the same in effectiveness.
A friend on Friday got vaccinated against the flu "Vaxigripp" now lies with a runny nose and a sore throat. Is this normal ??
Answers:
Vadim Kolosov
it means that there is no immunity at all, vaccination is a weak disease. Instead of vaccination, you need to temper and not smoke, of course, otherwise nothing will help
Regina
Yes it is quite normal reaction... You need to take the antihistamine Diazolin, Loratodin and an immunostimulant, for example Aflubin, and everything will go away and be fine!
Mila
Yes, it can be, or it’s a reaction to the vaccine. And the reaction can be like this. This is fine. And also, since the vaccine does not start to work immediately, the girl (just coincidentally) just got sick.
Mila
She apparently was already unhealthy at this point, this is the effect that happened or it may be that she was recently ill and did not recover, but in general, when the vaccine is administered, an infection occurs, but in a small dose so that antibodies can be produced, therefore it digests it the body is vaccinated is normal
"Sovigripp" (vaccine): reviews of doctors and patients. Description, instructions, contraindications
Well, today we will try to figure out what kind of "Sovigripp" (vaccine) reviews earns from doctors and patients. This drug is extremely important for modern man... After all, it is he who helps to fight viral infections whose epidemics are so common. True, before you enter the injection, you really need to find out what doctors and patients think about this. Maybe it's better to use old vaccines altogether? Or pay attention to a new one? Let's understand this issue. 
Description
Let's start with the fact that Sovigripp (vaccine) receives reviews from the moment of its description. That is, as soon as patients find out what it is, they write their opinions about the medication. This is not entirely correct. But the fact remains.
The point is that Sovigripp is a kind of new Russian flu vaccine. A kind of concentrated solution in ampoules that contains killed and weakened influenza bacteria different types... Honestly, there is nothing special about this. Only now "Sovigripp" (vaccine) does not receive consumer reviews itself better character already because it is domestic. The modern population is skeptical about Russian vaccines. Especially new ones. This is exactly what our today's drug is.
Indications
Of course, it is impossible to do without special indications for use. And this vaccine against influenza "Sovigripp", reviews of which will be presented to our attention a little later, has a number of both recommendations and contraindications. You need to pay close attention to them. After all, virus injections and vaccinations are no joke.
"Sovigripp" is a vaccine that is recommended for administration to the entire adult population wishing to increase their immunity and protect themselves from various types of influenza. Permitted for use by people over the age of 18. As the manufacturers assure, even during pregnancy and lactation (the most vulnerable categories of citizens, by the way), it is possible to vaccinate with the drug. "Sovigripp" (vaccine) is highly recommended, reviews of which are left ambiguous, for those who have reduced immunity, and there is also a risk of serious complications after an illness. Sometimes this remedy also applies when routine vaccinations from the flu in schools. But it applies exclusively to the senior level of schoolchildren (grades 8-11). 
Contraindications
Contraindications should not be forgotten either. It is not enough to want to protect yourself from viral diseases and flu. You also need to have an idea of when it is impossible to inject. Otherwise, you can simply harm own health... "Sovigripp" (vaccine), instructions, reviews and the effectiveness of which are presented to our attention, is not just some kind of injection. A really serious injection. And for this reason, contraindications should not be overlooked.
The flu vaccine "Sovigripp", reviews of which are of interest to many patients, is not recommended for use for people with hypersensitivity, as well as for those who generally have an intolerance to any vaccinations. Such cases are rare, but they do occur. Also with viral diseases, elevated temperature and any discomfort is banned from Sovigripp. Young children and junior schoolchildren should also not be given this injection. If you are prone to allergic reactions (including protein), you can also forget about the domestic method of protection against ARVI and influenza. 
The restrictions don't end there. Strong post-vaccination reactions of the body and chronic diseases in the patient are also reasons for refusing to vaccinate with the drug. Thus, we can conclude that the medication has more than enough contraindications. Do not be afraid - similar restrictions are imposed on most vaccines. After all, a person should get a weakened or killed virus when the body is in excellent condition. Otherwise, there is a risk not of developing immunity, but of disease.
Opinion of doctors
An important point is what doctors think about the domestic vaccine. It is not in vain that before any vaccination it is advisable to consult a specialist. The vaccine "Sovigripp" earns reviews of doctors, oddly enough, very good.
Many claim that this remedy Russian production helps to fight the flu and its viruses faster than others. In addition, doctors say that the risk of complications in this case is reduced to almost zero. Allegedly, almost all patients tolerate the injection calmly and without consequences. 
Plus, sometimes this drug assigned to children. So there is every reason to trust the vaccine. A pediatrician will never prescribe a drug that could harm a child. Yes, doctors also emphasize that for maximum effect I have to give Sovigripp injections every year. But this is a compulsory measure, and it applies to all flu shots. So there is nothing to be afraid of.
Patients say
But the opinion of patients does not always coincide with the words of doctors. The population is so arranged that domestic medicine in recent times there is no particular trust. And therefore, "Sovigripp" (vaccine) reviews of patients earns far from the best.
For example, the main reason for this is the manufacturer. He is Russian. And this fact already makes people doubt the effectiveness and safety of vaccination. It's no secret that in Russia, despite the development of the country, testing is not particularly effective. medical supplies... And therefore, the risk of complications after injections remains high. But all this is carefully hidden. After all, doctors' main task is to promote domestic medicine.
In addition, patients indeed repeatedly emphasize that after the vaccine, their general state health for a few days. All this is attributed to side effect drug. Only now these cases are encountered very often. And this does not please the population. Sometimes after vaccination, you can feel really sick. This is exactly what people who have already tried Sovigripp assure. 
Outcomes
What conclusion can be drawn from all this? "Sovigripp" (vaccine) reviews receive dubious and ambiguous. The opinions of doctors and patients were divided. And here everyone has the right to decide who to believe more. In principle, if you do not suffer from viral diseases, and are also not afraid of new drugs, then our today's option is quite suitable for an injection.
"Sovigripp" (vaccine), instructions, reviews, recommendations and contraindications of which were presented to our attention, is domestic product first of all. There is no need to be afraid of him. Yes, experimenting when you suffer from side effects is not worth it. And children do not need to inject such injections either, until this drug is considered a "new word" in medicine. Otherwise, there is no apparent reason for concern.
Last updated description by manufacturer 08.08.2007
Filtered list
ATX
Pharmacological group
3D images
Composition and form of release
| Suspension for intramuscular and subcutaneous administration | 1 dose (0.5 ml) |
| One dose of the vaccine (0.5 ml) contains hemagglutinin (HA) and neuraminidase (HA) of the following viral strains: | |
| A (H 3 N 2) | 15 μg HA |
| A (H 1 N 1) | 15 μg HA |
| B | 15 μg HA |
| Excipients: potassium chloride; potassium dihydrogen phosphate; sodium phosphate dihydrate; sodium chloride; calcium chloride dihydrate; magnesium chloride hexahydrate; water for injections |
in disposable syringes complete with 0.5 ml injection needles; in a pack of cardboard 1 or 10 sets.
Description of the dosage form
Transparent colorless liquid.
Characteristic
Influenza vaccine, subunit, inactivated... Influvac ® is a trivalent inactivated influenza vaccine consisting of surface antigens (HA, HA) of influenza A and B viruses grown on chicken embryos. The antigenic composition of the influenza vaccine is updated annually according to the WHO recommendations.
pharmachologic effect
pharmachologic effect- formative development of specific immunity.Pharmacodynamics
Forms specific immunity to influenza viruses of types A and B, which occurs, as a rule, 14 days after vaccination and lasts up to 1 year.
Indications for Influvac ® (subunit inactivated influenza vaccine)
Prevention of influenza in adults and children from 6 months.
persons over 65 years old, regardless of their state of health;
patients with respiratory diseases;
patients with cardiovascular diseases of any etiology;
patients with chronic renal failure;
patients with diabetes mellitus;
patients with immunodeficiency diseases (HIV infection, malignant blood diseases, etc.) and patients receiving immunosuppressants, cytostatics, radiation therapy or high doses of corticosteroids;
children and adolescents (from 6 months to 18 years old) who receive drugs containing acetylsalicylic acid for a long time, and, therefore, are at an increased risk of developing Reye's syndrome due to influenza infection;
pregnant women (II-III trimester). Pregnant women in high-risk categories should be vaccinated regardless of the stage of pregnancy.
Contraindications
hypersensitivity to chicken protein or any other component of the vaccine;
severe fever or allergic reactions after previous vaccination with subunit influenza vaccines.
Vaccination is postponed until the end of acute manifestations of the disease and exacerbation of chronic diseases. For mild ARVI, acute intestinal and other diseases, vaccination is carried out immediately after the temperature has returned to normal.
Application during pregnancy and lactation
Application experience shows that Influvac ® has no teratogenic or toxic effect on the fetus. Influvac ® vaccine can be used during lactation.
Side effects
On the part of the circulatory and lymphatic system: rarely, thrombocytopenia.
From the immune system: rarely - allergic reactions are possible; very rarely - anaphylactic shock.
From the nervous system: often - headache; rarely - paresthesia, convulsions, encephalomyelitis, neuritis, Guillain-Barré syndrome.
However, no convincing data have been established on the association of these reactions with vaccination.
From the vascular system: very rarely - vasculitis with transient renal dysfunction.
General disorders: often - fatigue and neuralgia, which do not require treatment and disappear in 1-2 days.
Local reactions: redness, swelling, soreness, induration, ecchymosis.
Systemic reactions: fever, malaise, tremors, sweating, soreness in muscles and joints.
In the room where vaccination is carried out, it is necessary to have drugs for the treatment of anaphylactic shock (adrenaline, glucocorticoids, etc.).
Interaction
Influvac ® can be used simultaneously with other vaccines (vaccines should be injected into different parts of the body with different syringes). Increased side effects are possible.
If the patient is receiving immunosuppressive therapy, the immune response may be reduced.
After vaccination, it is possible to receive false positives serological tests (when carrying out an enzyme-linked immunosorbent assay (ELISA), which are due to the production of IgM after vaccination.
Incompatibility: cases of incompatibility are unknown.
Method of administration and dosage
V / m or PC(deep). It is strictly forbidden to inject the drug intravenously. Immunization is carried out annually in the fall. Adults and adolescents (from 14 years old) - 0.5 ml once, children: from 6 months to 3 years old - 0.25 ml, from 3 to 14 years old - 0.5 ml once; children who have not previously had the flu and have not been vaccinated, as well as patients with immunodeficiency - twice with an interval of 4 weeks.
Precautionary measures
The drug may contain an undetectable residual amount of gentamicin, therefore, caution should be exercised when vaccinating individuals with hypersensitivity to aminoglycosides.
special instructions
The vaccine retains its properties for 12 months. The expiration date is considered to be June 30 of the year following the year of issue. Do not use after the expiration date stated on the package.
Directions for handling disposable syringes
The vaccine must warm to room temperature before being administered. Immediately before injection, the syringe must be shaken, remove the protective cap from the needle and remove air from the syringe, keeping it in an upright position with the needle up and slowly pressing the plunger. When a dose of 0.25 ml is administered, the movement of the syringe plunger is stopped at the moment when its inner surface reaches the lower edge of the needle retainer.
Manufacturer
Solvay Pharmaceuticals B.V., The Netherlands.
Storage conditions of Influvac ® (subunit inactivated influenza vaccine)
In a dark place at a temperature of 2-8 ° C (do not freeze).Keep out of the reach of children.
Shelf life of Influvac ® (subunit inactivated influenza vaccine)
12 monthsDo not use after the expiration date printed on the package.
