Organization of intra-pharmacy production of medicines Subject-quantitative accounting of medicines
For weighing and cooking medicines containing poisonous and narcotic substances, it is necessary to use separate hand weights, weights, mortars, cylinders, funnels, etc. These items should be stored in cabinet A. , "For mercury dichloride", etc. The dishes used for the preparation of medicines containing poisonous and narcotic substances are washed and processed separately from other dishes under the supervision of a pharmacist. Mortars with a powder or ointment mass before hanging, as well as funnels when filtering solutions, are covered with a glass or plastic roof.
Before starting the manufacture of medicines, the pharmacist must carefully read the prescription, check the compatibility of the prescribed ingredients and the correctness of the indicated doses. Particular attention must be paid to the manufacture of medicines containing poisonous, narcotic and potent substances... Poisonous and narcotic substances included in the composition of the medicinal product must be weighed by the pharmacist-technologist at the place of their storage in the presence of the pharmacist, after which the bar is immediately placed in cabinet A., on the back of the prescription, the pharmacist-technologist signs for the issue, and the pharmacist - on receipt the required amount poisonous or narcotic substance indicating its name and quantity. After receiving a poisonous substance, the pharmacist must immediately use it for the manufacture of a medicinal product. The prepared medicinal product is immediately transferred to the pharmacist-technologist (pharmacist-analyst) for control, after which it is stored in a special cabinet. If one pharmacist works in the pharmacy, then after weighing the poisonous and narcotic substance, he independently indicates on the back of the prescription the name and the amount of the substance taken in words and puts his signature.
Organization of subject-quantitative accounting (PKU) pr. No. 785 (list of drugs)
All recipes containing substances on the PCU are subject to daily sampling. At the end of the day, pharmacist-technologist, head. the prescription-production department or another responsible person selects all the recipes, puts them according to the name of the drug, and a daily sample sheet is drawn up for each name: - indication of the name; - the date; - quantity in gr. consumed drugs; - the signature of the pharmacist.
Based on the selected recipes and requirements of the medical facility head. the prescription-production department draws up a special journal "Journal of registration of operations related to the circulation of narcotic, psychotropic and other substances located in the PKU. (Legislation governing accounting - the federal law"On narcotic drugs and psychotropic substances" - pr. Ministry of Health of the Russian Federation No. 330 of November 1997; pr. Ministry of Health of the Russian Federation No. 205 of May 2005; pr. MZ RT No. 1598 dated October 2003). In this magazine, each drug name is assigned a separate page. Recorded monthly: - arrival within a month of LP; - where it was received from, on the basis of which document; - the number and series of medicinal products; - date and signature of the person responsible for receiving the medicinal product.
According to the accounts on the basis of daily sample sheets, the consumption of drugs is recorded: - with division - sales and dispensing; - total consumption per month; - book balance, actual balance; - signature of the financially responsible person and date.
At the end of the month, the final actual balance is carried over and becomes the initial one. Consumption with indication of the series. Vacation - Angro - in health care facilities in the buyer's dishes.
Final book balance = rest. final month + income - expense
Final actual remainder = weighing the rod - m of the rod itself = m LV
The magazine is a document of special accounting, its pages are numbered, the sheets are laced, on the last page it is sealed with a wax seal and the number of pages is indicated, signed by the head of the higher organization and stamped.
Organization of intra-pharmacy quality control of medicines in a pharmacy. Organization of the workplace of the pharmacist-analyst, with his tasks. Mandatory types of intra-pharmacy quality control.
Separate requirements are imposed on production facilities, in particular assistant, and especially aseptic, etc. For a rational organization production processes the assistant's room should be connected with the recipe and the control and analytical room. Close to entrance doors sinks with cold and hot water, and pipelines with distilled water or distilled water for injection should be connected to the workplaces. In these rooms, along with general ventilation, household electric air cleaners or air conditioners are installed to clean the air.
To provide correct storage, manufacturing, quality control and dispensing of medicines, approximate standards for technical and economic equipment of self-supporting pharmacies have been approved. The norms provide for equipping pharmacy premises with furniture for storing medicines, dressings and other products medical purpose, means of small mechanization, technological equipment, electrical appliances, as well as production and household equipment.
TO technological equipment and the means of mechanization used in pharmacies in the course of production activities include: apparatus for obtaining and storing distilled water and water for injection, technical means for sanitizing dishes, medical sterilization apparatus, electric mixing devices, weighing devices, an installation for the production of ointments, capping devices, various dispensers for powder and liquid medicines, apparatus for filtering solutions, etc. To ensure quality control of medicines, pharmacies are equipped with refractometers, photoelectric calorimeters, potentiometers, pH meters, microscopes and other devices.
Once a quarter, compliance with the rules for storing drugs is monitored, there are also mandatory types of intra-pharmacy quality control:
Written control;
Survey control;
Organoleptic control;
Chemical control;
Vacation control.
8. Pharmaceutical expertise of prescriptions. General rules for writing out and assigning prescriptions. The current regulatory framework. Technology for receiving, registering and accounting for custom-made recipes. Recipe taxation rules. The essence of vacation control.
Pharmaceutical prescription expertise - this is the determination of the conformity of the received recipe current regulations writing prescriptions.
It is forbidden to write out prescriptions:
for medicinal products not approved for medical use;
in the absence of medical indications;
for medicines used only in medical institutions;
When receiving prescriptions and dispensing drugs on them, the conformity of the form of the prescription form of the medicinal prescription, the validity of the prescription, the presence of mandatory requisites, the presence of additional requisites, the eligibility of the person who wrote the prescription, the correctness of the prescription and the method of application, compatibility of the ingredients in the recipe, single doses, maximum dosage rates of drugs for which they are established.
The attending physicians conducting outpatient appointments are entitled to prescribe all types of drugs. Dentists, paramedics, midwives write out prescriptions for medicines to patients with their signature and with an indication of their medical rank only in special cases. They, as well as private practitioners, are prohibited from prescribing drugs of lists II and III.
When prescribing NS or PV lists II and III, other drugs subject to PKU, the dose of which exceeds the highest single dose, the doctor must write the dose in writing and put an exclamation mark.
Only permitted Latin abbreviations are allowed. It is forbidden to be limited to general instructions: "Internal", "Known". Only allowed adopted by the rules abbreviations of designations. Corrections in the recipe are not allowed.
The rates for prescribing and dispensing Schedule II narcotic drugs, barbituric acid derivatives, and other drugs subject to PCU for incurable oncological and hematological patients may be doubled compared to the approved amount. When prescribing narcotic drugs for which the dispensing rate is not provided, their maximum permissible amount for prescription in one prescription can be five times higher than a single dose specified in the instructions for medical use.
When writing prescriptions for chronically ill physicians, prescriptions are allowed to be valid for up to one year. The doctor must make a note "Chronic patient", indicate the validity period of the prescription and the frequency of dispensing drugs from the pharmacy, certify this indication with his signature and personal seal, as well as the seal of the medical and prophylactic institution "For prescriptions".
A prescription that does not meet at least one of the listed requirements or contains incompatible medicinal substances is considered invalid. All incorrectly written prescriptions are canceled with the stamp “Prescription invalid” and are recorded in the journal of incorrectly written prescriptions. Information about health workers who write out prescriptions incorrectly is presented to the heads of the respective health care facilities.
Forms of prescription forms, their purpose and the procedure for filling out are regulated by the order of the Ministry of Health of the Russian Federation No. 110 dated 12.02.2007 "On the procedure for prescribing and prescribing medicines, medical products and specialized health food products." The procedure for dispensing drugs is regulated by order of the Ministry of Health of the Russian Federation No. 785 dated 14.12.2005 “On the procedure for dispensing drugs”. The list of NS, PV and P was approved by the RF Government Resolution No. 681 of June 30, 1998 "On approval of the list of NS, PV and P subject to control in the Russian Federation."
Prescription, written correctly, taxed, that is, its retail price is determined. The retail price for extemporal dosage forms and intra-pharmaceutical preparation consists of the cost of the initial ingredients, the cost of the pharmaceutical tableware and the tariff for the manufacture of the medicine. Pharmacies - legal entities have the right to independently develop tariffs for the manufacture and packaging of drugs. Tariffs must be approved by the order of the pharmacy. Tariffication is based on: standards of time spent on individual operations for the manufacture, control, packaging and dispensing of extemporal dosage forms and intra-pharmaceutical preparations.
Registration of recipes. For recipes requiring custom production, registration can be done in a variety of ways. The most common is receipt form registration of recipes. The receipt is filled in one copy when accepting prescriptions. Conventionally, it can be divided into three parts. The first part of the receipt indicating the number of the medicine, the patient's name, cost and LF remains in the pharmacy. The second part of the receipt indicating the number of the drug, the type of dosage form, the name of the patient, the date and time of manufacture of the drug, and its cost is handed over to the customer.
The third part of the receipt contains two identical numbers: the first with the indication "prepared", "checked", "released" is pasted on the prescription, the second - on the packaging of the dispensed medicine.
Accepted prescriptions for custom-made medicines can be registered with recipe magazine, containing the following data: date, prescription number, name of the patient, his address and telephone number, LF, cost of the medicine.
Control during dispensing is regulated by order of the Ministry of Health of the Russian Federation No. 214 dated July 16, 1997 "On quality control of drugs manufactured at JSC". Vacation control - control that all drugs produced in pharmacies are subjected to, while checking the compliance of the drug packaging with the physicochemical properties of the ingredients included in them, the doses of poisonous, narcotic or potent drugs indicated in the prescription With the patient's age, numbers on the prescription and numbers on the label, the patient's name on the receipt, surnames on the label and the recipe or its copies, copies of recipes, recipes, registration of drugs in accordance with current requirements.
On vacation Special attention applies for registration with the appropriate warning notices of the LF. On the labels of drugs made in pharmacies for medical institutions, the composition of the drug, the number of the medical institution, the name of the department (office), the analysis number, and the expiration date are indicated.
The person who released the drug is obliged to put his signature on back side prescription (requirements).
9. The procedure for prescribing, discharging and dispensing NS, PV, SD and JV from the pharmacy. Organization of the PKU. The procedure for admitting specialists to work with NS and PV. Accounting for the movement of NS and PV in the pharmacy. Legal and regulatory framework. Vacation rates, the procedure for attaching patients to the pharmacy.
According to Federal Law No. 3 dated 01/08/1998 "On narcotic drugs and psychotropic substances", narcotic drugs are substances of synthetic or natural origin, drugs included in the List of NS, PV and their P subject to control in the Russian Federation, in accordance with the legislation of the Russian Federation , international treaties of the Russian Federation, including the 1961 Single Convention on the National Assembly.
Psychotropic substances - substances of synthetic or natural origin, drugs, natural materials included in the List of HC, PV and their P subject to control in the Russian Federation, in accordance with the legislation of the Russian Federation, international treaties of the Russian Federation, including the 1971 Convention on PoE.
Precursors are substances that are often used in the production, manufacture, processing of HC and PS, included in the List of HC, PS and their P subject to control in the Russian Federation, in accordance with the legislation of the Russian Federation, international treaties of the Russian Federation, including the UN Convention against Illegal turnover of NS and PV in 1988.
This law, depending on the control measures applied by the state, distributes the National Assembly, PoE and P to Lists I, II, II and IV. The list of NS, PV and P was approved by the RF Government Resolution No. 681 of June 30, 1998 "On approval of the list of NS, PV and P subject to control in the Russian Federation." The SDYAV lists were approved by Decree of the Government of the Russian Federation No. 964 of December 29, 2007.
The release of narcotic drugs and psychotropic substances to individuals is carried out only in pharmacy organizations and health care institutions if they have a license for the specified type of activity.
For medical purposes, narcotic drugs and psychotropic substances included in lists II and III can be used. They are available with a prescription. The use of narcotic drugs and psychotropic substances included in lists II and III in the medical activities of private practitioners is not allowed.
When prescribing narcotic drugs and psychotropic substances included in lists II and III, the attending physician must interview the patient about previous prescriptions of narcotic drugs and psychotropic substances and make an appropriate entry in the medical documents.
The form "Special prescription form for a narcotic drug and psychotropic substance" is made on pink paper with watermarks and has a serial number. On the prescription form in the upper left corner, a stamp of a medical organization is put down with an indication of its name, address and telephone number. The prescription form is completed legibly by the doctor, in ink or ballpoint pen.
The column "Rp:" indicates Latin the name of the medicinal product, its dosage. In the column "Reception" the method of application is indicated. In the column "Gr." the full surname, name, patronymic of the patient is indicated. In the column "Case history N" the number of the outpatient's card is indicated. The full surname, name, patronymic of the doctor is indicated.
The prescription is signed by the doctor and certified by his personal seal. In addition, the prescription is signed by the chief physician of the medical organization or his deputy and certified by the round seal of the medical organization.
Only one name is written on one prescription form. Corrections are not allowed.
Pharmacy organizations and health care institutions are prohibited from dispensing narcotic drugs and psychotropic substances included in Schedule II on a prescription issued more than five days ago. Prescriptions for psychotropic substances in Schedule III are valid for 10 days.
The outer packaging of narcotic drugs and psychotropic substances must exclude the possibility of their extraction without violating the integrity of the said packaging. The inner packaging of narcotic drugs and psychotropic substances used for medical purposes must be marked with a double red stripe.
Regulated by the order of the Ministry of Health of the Russian Federation No. 785 dated December 14, 2005 "On the procedure for dispensing drugs."
Medicines containing narcotic drugs, psychotropic substances and their precursors and included in the List of Medicines sold without a doctor's prescription must be dispensed by pharmacies in an amount of no more than 2 packages to the consumer.
The PKU is kept in the "Book of accounting for the NS and other drugs subject to the PKU", numbered, laced, sealed and certified by the signature and seal of the head. The book starts up for 1 year.
Prescriptions for HC and PoE in Schedule II and PS in Schedule III are kept in the pharmacy for ten years.
In AO, the leave of NS and PV included in List II of the List is carried out by patients attached to a specific outpatient clinic, which is assigned to the AO. The assignment of an outpatient clinic to a JSC can be carried out by the health care or pharmaceutical management body of the constituent entity of the Russian Federation in agreement with the territorial body for control over the circulation of NS and PV.
Upon receipt of a prescription for a custom-made drug prescription, a pharmaceutical worker of AO is obliged to dispense a drug subject to PKU in half of the highest single dose in the event that a doctor prescribes drugs in a dose exceeding the highest single dose.
In accordance with the Decree of the Government of the Russian Federation No. 644 of 04.11.2006 "On the procedure for submitting information on activities related to the circulation of NS and PV ..." JSCs submit an annual report on the amount of manufactured, released and sold NS and PV, using for this a special register of transactions related to the circulation of NS and PV.
10. The order of dispensing from the pharmacy organizations of poisonous substances, ethyl alcohol and alcohol-containing solutions. Organization of storage of NS and PV. Subject-quantitative accounting. Release of codeine-containing drugs. Legal and regulatory framework.
The SDYAV lists are approved by Decree of the Government of the Russian Federation No. 964 dated December 29, 2007. If a substance from of this list is in the list of substances subject to PKU, it is released on prescription forms of form N 148-1 / y-88. The shelf life of such prescriptions in a pharmacy is three years. If it is not subject to PKU, but refers to prescription substances, then it is released on form N 107-1 / y. If this is a drug over-the-counter leave, then it is dispensed without a prescription.
Vacation ethyl alcohol, according to the order of the Ministry of Health of the Russian Federation No. 785 of 12/14/2005 "On the procedure for dispensing drugs", the following is carried out:
according to recipes written out with the inscription "For applying compresses" (indicating the required dilution with water) or "For skin treatment" - up to 50 grams per pure form;
according to prescriptions written for a medicinal prescription of individual manufacture - up to 50 grams in a mixture;
according to prescriptions issued for an individual drug prescription, with the inscription "By special purpose", separately certified by the signature of the doctor and the seal of the medical institution" For prescriptions ", for patients with a chronic course of the disease - up to 100 grams in mixture and in pure form.
Ethyl alcohol is dispensed on forms N 148-1 / y-88, as it is subject to PKU.
The storage rules for NS, PV and P are regulated by the Decree of the Government of the Russian Federation No. 1148 dated December 31, 2009 "On the procedure for storing NS, PV and P". Storage of NS, PV and P is carried out in isolated rooms, specially equipped with engineering and technical means of protection. The premises are divided into 4 categories. TO 1st category includes the premises of manufacturers and manufacturers (with the exception of pharmacies) NS, PV and P, intended for storage raw materials and finished products, as well as the premises of organizations engaged in the wholesale trade of NS, PV and P and their processing ... To the 2nd category include AO premises intended for storing a monthly supply of HC and PV used for medical purposes. To the 3rd category includes the premises of health care institutions intended for storing a 5-day and (or) 3-day supply of NS and PV and NS and PV, handed over by the relatives of deceased patients, premises of legal entities intended for storing NS and PV used in veterinary, scientific, educational and expert purposes. To the 4th category includes the premises of health care institutions intended for storing the daily supply of NS and PV, as well as places for temporary storage of NS and PV used for medical purposes (ambulances and emergency medical services, posts of paramedical personnel, assistant rooms of pharmacies, etc.).
Subject-quantitative accounting regulated by the order of the Ministry of Health of the Russian Federation No. 785 dated December 14, 2005 “On the procedure for dispensing drugs”. The PKU is kept in the "Book of accounting for the NS and other drugs subject to the PKU", numbered, laced, sealed and certified by the signature and seal of the head. The book starts up for 1 year.
Codeine and codeine-containing drugs are included in Schedule II, according to the RF Government Decree No. 681 of June 30, 1998 “On approval of the list of NS, PV and P subject to control in the RF”. According to the order of the Ministry of Health of the Russian Federation No. 110 of 12.02.2007 "On the procedure for prescribing and prescribing drugs, medical devices and specialized products health food», They must be written out on special prescription forms for a narcotic drug and a psychotropic substance. The prescription is valid for 5 days from the date of issue. Maximum concentration limit of codeine for one recipe is 0.2 g of powder. MPC for combined medicinal products containing codeine (codeine phosphate) in tablets, capsules, solutions, etc. - no more than 0.2 g in terms of pure substance.
When writing a prescription for a custom-made medicinal prescription containing codeine in a dose not exceeding the highest single dose, and provided that this combined drug is not a Schedule II narcotic or psychotropic substance, form No. 148-1 / y should be used. 88.
11. Inventory department at the pharmacy. States. Department tasks. Organization of acceptance of medicines and other groups of pharmaceutical products in a pharmacy. Organization of storage in accordance with the current regulatory framework. Laboratory packing work in a pharmacy. Formation of prices for intra-pharmacy preparation and packaging.
According to the organizational structure, the pharmacy can be organized stock department... The department is headed by a pharmacist. The department employs pharmacists-technologists and packers who are subordinate to the head and his deputies.
If such a department is not provided in a pharmacy, then stocks of medical goods are under the jurisdiction of the prescription-production department or directly by the head of the pharmacy.
The department of stocks determines the current need of the pharmacy for the necessary drugs and medical devices, timely submission of orders-requests to pharmaceutical warehouses and other supply bases, accepting incoming drugs, ensuring their storage, as well as dispensing medicines and medical devices to other departments, small retail chains and health care facilities.
The inventory department includes the following premises: unpacking, storerooms, premises for servicing medical facilities. The department can carry out laboratory and packaging work. For their implementation, a room is allocated (defect room).
Acceptance requirements regulated by OST 91500.05.0007-2003 "Rules for dispensing drugs in pharmacy organizations. Basic provisions". When carrying out loading and unloading operations during the acceptance or shipment of medicinal products and other goods, the incoming drugs must be protected from precipitation, exposure to low and high temperatures.
Preparations and other expired products that do not meet quality requirements, standards and without documents certifying their quality are not subject to acceptance.
For drugs in damaged packaging that do not have certificates and / or the necessary accompanying documentation, rejected when receiving or dispensing to the patient, not corresponding to the order or with an expired shelf life, an act is drawn up. They must be appropriately marked and placed separately in a designated area until they are identified, returned to the supplier or destroyed.
Narcotic drugs, psychotropic substances, thermolabile medications must be placed in storage immediately. All deliveries must be accompanied by documents allowing to establish the date of shipment, the name of the medicinal product, the batch and batch number, the quantity of goods, the price of the drug, the name and address of the supplier and recipient, as well as documents confirming the quality of the drugs.
Storage of drugs organized in accordance with the order of the Ministry of Health of the Russian Federation No. 706n dated 23.08.2010 "On approval of the Rules for the storage of medicines."
The order establishes general requirements for rooms for storing drugs and organizing their storage, requirements for rooms for storing flammable and explosive drugs, specifics of organizing drug storage in warehouses, regulates storage of drugs that require protection from light, moisture, and volatilization and drying, from exposure to increased or low temperature, from exposure to gases contained in the environment, storage of odorous and coloring drugs, storage of disinfecting drugs, storage of drugs for medical use, storage of medicinal products, storage of medical leeches, storage of flammable and explosive drugs, storage of SDYAV and drugs subject to PKU.
The storage rules for NS, PV and P are regulated by the Decree of the Government of the Russian Federation No. 1148 dated December 31, 2009 "On the procedure for storing NS, PV and P". Storage of NS, PV and P is carried out in isolated rooms, specially equipped with engineering and technical means of protection. The premises are divided into 4 categories.
Storage of medical devices is regulated by the order of the Ministry of Health of the Russian Federation No. 1198n dated December 27, 2011 "On the approval of the rules in the field of circulation of medical devices". According to the order, the storage of medical products in the JSC is carried out by groups: rubber products, plastic products, dressings and auxiliary materials and other medical products.
The manufacture of concentrates, semi-finished products and intra-pharmaceutical preparations in a pharmacy is called laboratory work, and intra-pharmacy packaging - packing works. Intra-pharmacy preparation- This is a preliminary preparation of dosage forms according to frequently occurring prescription recipes. Intra-pharmacy packing- dosage of drugs in quantities suitable for dispensing to customers. Concentrates, semi-finished products and VAZ are prepared under aseptic conditions and must undergo complete chemical control.
Laboratory and packing works are recorded in the Register of laboratory and packing works according to the approved form. The magazine must be numbered, laced and signed by the head of the JSC. In large pharmacies, laboratory and packaging works are kept separately.
The journal is used for accounting and control over the performance of laboratory and packing work, for posting or writing off amounts at the cost of medicines and manufactured products put into operation or the results of rounding prices per packing unit, etc. The journal also takes into account the cost and quantity of pure ethyl alcohol dispensed to the population according to prescriptions. The price of an intra-pharmacy drug consists of the cost of medicinal ingredients, pharmaceutical utensils, auxiliary materials and the tariff for the manufacture of the drug.
The procedure for maintaining the Register of laboratory and packaging works... Column 4 of the magazine shows all the ingredients received for the internal pharmaceutical preparation, column 7 indicates the retail price of medical goods and utensils issued for packaging, and column 14 - the actual retail price per unit of packaging of finished products, based on the retail cost of medicines, packaging, tariff, etc. At the end of the month, the amounts are calculated in columns 8 and 15, and the difference (rounding result) is shown in columns 19 and 20 for each laboratory work or type of packaging.
When performing laboratory packing work, there may be a difference between the cost of medicines, utensils, water issued for work, tariffs for manufacturing and the cost of manufactured products. This difference results from rounding prices. If the finished product is more expensive, then an additional estimate is formed. If the finished product turns out to be cheaper than the original ingredients, water and tariffs, then a markdown is formed. The amount of the markdown is written off as an expense for distribution costs.
12. The procedure for providing the population with the necessary medicines (DLO-ONLS). Beneficial categories of citizens eligible for state social assistance. Procedure for the implementation of drug supply programs for “territorial” beneficiaries. Legal and regulatory framework. Current lists of medicines. Purchase and registration of the movement of medicines in the system of preferential sales.
13. Organization of intra-pharmacy quality control of individually manufactured drugs. Types of quality control. The list of specialists who know the types of intra-pharmacy quality control. Documenting. Equipping the workplace of a chemist-analyst and a chemist-technologist for intra-pharmacy quality control (internal controller). Their functional and job responsibilities. Regulations.
Intra-pharmacy quality control is regulated by order of the Ministry of Health of the Russian Federation No. 214 dated July 16, 1997 "On quality control of drugs manufactured in JSC".
A pharmacist appointed to the position to control the quality of drugs manufactured in pharmacies (pharmacist-analyst) must be familiar with all types of intra-pharmacy control. To carry out chemical quality control of medicines manufactured in pharmacies, a special workplace must be equipped with a standard set of equipment, instruments and reagents, as well as provided with regulatory documents, reference literature. The results of quality control of medicines are registered in journals using the attached forms. All magazines must be laced, the pages in them must be numbered, certified by the signature of the head and the seal of the pharmacy. The logs are stored for one year. A report on the work on quality control of medicines manufactured in a pharmacy is drawn up based on the results for the year and sent to the territorial control and analytical laboratory.
Acceptance control consists in checking the incoming drugs for compliance with the requirements for the indicators "Description", "Packaging", "Marking", in checking the correctness of the settlement documents, as well as the availability of documents confirming the quality of drugs.
Written control consists in filling the PPK. The passport must indicate: the date of manufacture, the number of the prescription (number of the medical organization, the name of the department), the name of the drugs taken and their quantity, the number of doses, the signatures of the manufacturer, packaging and checking. All calculations must be made before the manufacture of the dosage form and recorded on the back. The passport is filled in immediately after production, from memory, in Latin, in accordance with the sequence of technological operations. PPKs are stored in the pharmacy for two months.
Manufactured drugs, recipes and completed passports are submitted for verification to a pharmacist performing control functions (pharmacist-technologist). The control consists in checking the compliance of the entries in the PPK with the prescription in the recipe, the correctness of the calculations.
Survey control applied selectively. It is carried out after the manufacture of no more than five dosage forms by the pharmacist. When conducting a survey control, the pharmacist-technologist names the first substance included in the dosage form, and in dosage forms of a complex composition also indicates its amount, after which the pharmacist names all the medicinal substances taken and their quantities.
Organoleptic control consists in checking the LF according to the indicators: "Description", homogeneity, absence of visible mechanical inclusions.
Physical control consists in checking the total mass or volume of the dosage form, the number and mass of individual doses (at least three doses) included in this dosage form.
Each batch of packaging and intra-pharmaceutical preparation is checked in the amount of at least three packages, dosage forms made according to individual recipes are checked selectively, at least 3% of the amount of DF made per day, each batch of DF requiring sterilization. When checking dosage forms, the quality of the closure is also monitored.

The list of drugs subject to quantitative accounting ORDER of the Ministry of Health of the Russian Federation of April 22, 2014 N 183 n Medicines - pharmaceutical substances and drugs containing narcotic drugs, psychotropic substances and their precursors (their salts, isomers, stereoisomers) included in lists II , III, IV list of narcotic drugs, psychotropic substances and their precursors subject to control in the Russian Federation, in combination with pharmacologically inactive substances, as well as drugs containing narcotic drugs, psychotropic substances and their precursors in combination with pharmacologically active substances (subject to inclusion them in the list as a separate item) Medicines - pharmaceutical substances and medicinal products containing narcotic drugs, psychotropic substances and their precursors (their salts, isomers, stereoisomers) included in lists II, III, IV of the list of narcotic drugs, psychotropic substances and their precursors, subject to control in the Russian Federation, in combination with pharmacologically inactive substances, as well as medicinal products containing narcotic drugs, psychotropic substances and their precursors in combination with pharmacologically active substances (subject to their inclusion in the list as a separate item)

The list of drugs subject to quantitative accounting ORDER of the Ministry of Health of the Russian Federation of April 22, 2014 N 183 n Medicines - pharmaceutical substances and drugs containing potent and toxic substances (their salts, isomers, ethers and esters, mixtures and solutions, regardless of concentration ) included in the lists of potent and toxic substances, in combination with pharmacologically inactive substances, as well as medicinal products containing potent and toxic substances in combination with pharmacologically active substances (subject to their inclusion in the list as a separate item) Medicines - pharmaceutical substances and medicinal products, containing potent and poisonous substances (their salts, isomers, ethers and esters, mixtures and solutions, regardless of concentration) included in the lists of potent and toxic substances, in combination with pharmacologically inactive substances, as well as drugs containing potent and toxic substances in combination with pharmacologically active substances (subject to their inclusion in the list as a separate item)

List of drugs subject to quantitative accounting ORDER of the Ministry of Health of the Russian Federation of April 22, 2014 N 183 n Combined medicinal products containing, in addition to small amounts of narcotic drugs, psychotropic substances and their precursors, other pharmacological active substances(Clause 5 of the Order of the Ministry of Health of the Russian Federation of May 17, 2012 N 562 n Combined medicinal products containing, in addition to small amounts of narcotic drugs, psychotropic substances and their precursors, other pharmacological active substances (Clause 5 of the Order of the Ministry of Health of the Russian Federation of May 17, 2012 N 562 n

ORDER of June 17, 2013 N 378 n On approval of the rules for registering transactions related to the circulation of drugs for medical use included in the list of drugs for medical use subject to quantitative accounting in special journals of transactions related to the circulation of drugs for medical use application, and rules for maintaining and storing special journals of operations related to the circulation of drugs for medical use

Forms of accounting journals Journal of registration of transactions related to the turnover of narcotic drugs and psychotropic substances Journal of registration of transactions related to the circulation of narcotic drugs and psychotropic substances Journal of registration of operations in which the number of precursors of narcotic drugs and psychotropic substances changes. of narcotic drugs and psychotropic substances Log of operations related to the circulation of drugs for medical use Log of operations related to the circulation of drugs for medical use

Resolution of the Government of the Russian Federation dated N 644 "On the procedure for submitting information on activities related to the circulation of narcotic drugs and psychotropic substances, and registration of transactions related to the circulation of narcotic drugs, psychotropic substances and their precursors" drugs and psychotropic substances

The register of transactions related to the circulation of narcotic drugs and psychotropic substances Legal entities, as well as their divisions carrying out activities related to the circulation of narcotic drugs, psychotropic substances, are required to keep registration logs in the forms in accordance with Appendix No. 1. carrying out activities related to the circulation of narcotic drugs, psychotropic substances are obliged to keep registration logs in the forms according to Appendix No. 1 Registration of transactions related to the circulation of narcotic drugs, psychotropic substances is carried out for each name of the narcotic drug, psychotropic substance on a separate expanded sheet of the registration log or in a separate registration log. Registration of transactions related to the circulation of narcotic drugs, psychotropic substances is kept for each name of a narcotic drug, psychotropic substance on a separate expanded sheet of the registration log or in a separate logbook. Any operations resulting in a change in the quantity and state of narcotic drugs, psychotropic substances are subject to entry in the registration log. Any operations resulting in a change in the quantity and state of narcotic drugs, psychotropic substances are subject to entry in the registration log.

Log book of transactions related to the circulation of narcotic drugs and psychotropic substances Log books must be bound, numbered and signed by the head legal entity and the seal of the legal entity. Registration logs must be bound, numbered and sealed with the signature of the head of the legal entity and the seal of the legal entity. If necessary, by the decision of the executive authority of the constituent entity of the Russian Federation, the body certifying the registration log is determined. If necessary, by the decision of the executive body of the constituent entity of the Russian Federation, the body certifying the registration log is determined. The head of a legal entity appoints persons responsible for maintaining and storing registration logs, including in subdivisions. The head of a legal entity appoints persons responsible for maintaining and storing registration logs, including in subdivisions. Entries in the registration logs are made by the person responsible for their maintenance and storage, with a ballpoint pen in chronological order immediately after each operation for each item on the basis of documents confirming the completion of this operation. Records in the registration logs are made by the person responsible for their maintenance and storage, ballpoint with a pen in chronological order immediately after each operation for each item on the basis of documents confirming the completion of this operation.

Journal of registration of transactions related to the circulation of narcotic drugs and psychotropic substances Documents or their copies confirming the performance of an operation with a narcotic drug, psychotropic substance, certified in accordance with the established procedure, are filed in a separate folder, which is kept together with the corresponding registration journal. Documents or their copies, confirming the commission of an operation with a narcotic drug, psychotropic substance, certified in accordance with the established procedure, are filed in a separate folder, which is stored together with the corresponding registration log. The numbering of entries in the registration logs for each name of a narcotic drug, psychotropic substance is carried out within a calendar year in ascending order of numbers. The numbering of entries in new registration logs begins with the number following last issue in the completed journals. The numbering of entries in the registration journals for each name of a narcotic drug, psychotropic substance is carried out within a calendar year in ascending order of numbers. The numbering of entries in new logs begins with the number following the last number in the filled logs. Log pages not used in the current calendar year are crossed out and not used in the next calendar year. Log pages not used in the current calendar year are crossed out and not used in the next calendar year.

Register of transactions related to the circulation of narcotic drugs and psychotropic substances An entry in the register of each operation carried out is certified by the signature of the person responsible for their maintenance and storage, indicating the surname and initials. The entry in the register of each transaction is certified by the signature of the person responsible for them maintenance and storage, indicating the surname and initials. Corrections in the registration logs are certified by the signature of the person responsible for their maintenance and storage. Erasures and uncertified corrections in the logs are not allowed. Corrections in the logs are certified by the signature of the person responsible for their maintenance and storage. Erasures and uncertified corrections in the logs are not permitted. Legal entities monthly conduct an inventory of narcotic drugs and psychotropic substances in the prescribed manner. Legal entities monthly conduct an inventory of narcotic drugs and psychotropic substances in accordance with the established procedure. The registration logs must reflect the results of the inventory of narcotic drugs and psychotropic substances. The registration logs must reflect the results of the inventory of narcotic drugs and psychotropic substances.

Journal of registration of transactions related to the circulation of narcotic drugs and psychotropic substances. The journal of registration of narcotic drugs and psychotropic substances is stored in a metal cabinet (safe) in a technically fortified room. The keys to the metal cabinet (safe) and the technically fortified room are held by the person responsible for maintaining and storing the registration journal. The register of narcotic drugs and psychotropic substances is kept in a metal cabinet (safe) in the technically fortified room. The keys to the metal cabinet (safe) and the technically fortified premises are held by the person responsible for maintaining and storing the registration log. On the basis of entries in the corresponding registration log, legal entities submit, in the prescribed manner, reports on activities related to the circulation of narcotic drugs and psychotropic substances.On the basis of entries in the corresponding registration log, legal entities submit, in accordance with the established procedure, reports on activities related to the circulation of narcotic drugs and psychotropic substances

The register of transactions related to the circulation of narcotic drugs and psychotropic substances The completed registration logs, together with documents confirming the implementation of transactions related to the circulation of narcotic drugs, psychotropic substances, are handed over to the archive of the legal entity, where they are stored for 10 years after the last entry was made in them The completed registration logs, together with documents confirming the implementation of transactions related to the circulation of narcotic drugs, psychotropic substances, are handed over to the archive of the legal entity, where they are stored for 10 years after the last entry was made in them. After expiration the specified period registration logs are subject to destruction according to the act approved by the head of the legal entity. After the specified period, the registration logs are subject to destruction according to the act approved by the head of the legal entity.

DECISION OF THE GOVERNMENT OF THE RUSSIAN FEDERATION of June 9, 2010 N 419 ON SUBMISSION OF INFORMATION ABOUT ACTIVITIES RELATED TO THE TURNOVER OF NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES PRECURSORS, AND REGISTRATION OF OPERATIONS RELATED TO THEIR SPECIAL TURNOVER REGISTERS drugs and psychotropic substances included in List IV of the List of narcotic drugs, psychotropic substances and their precursors subject to control in the Russian Federation, approved by Decree of the Government of the Russian Federation of June 30, 1998 N 681 drugs and psychotropic substances included in List IV of the List of narcotic drugs, psychotropic substances and their precursors subject to control in the Russian Federation, approved by Decree of the Government of the Russian Federation of June 30, 1998 N 681

The procedure for maintaining and storing a log of operations in which the number of precursors changes When carrying out activities related to the turnover of precursors, any operations in which the number of precursors changes are subject to entry in a special log; When carrying out activities related to the turnover of precursors, any operations at which the number of precursors changes are subject to entry in a special journal; Operations are recorded for each precursor name on a separate expanded journal sheet or in a separate journal; Operations are recorded for each precursor name on a separate expanded journal sheet or in a separate journal; The magazine must be bound, numbered, certified by the signature of the head of the legal entity and sealed with the seal of the legal entity; The magazine must be bound, numbered, certified by the signature of the head of the legal entity and sealed with the seal of the legal entity; The head of a legal entity or an individual entrepreneur appoints persons responsible for maintaining and storing the journals; the head of a legal entity or an individual entrepreneur shall appoint persons responsible for maintaining and storing the journals.

The procedure for maintaining and storing the journal The numbering of entries in the journals for each name of the precursor is carried out within a calendar year in ascending order of numbers. The numbering of entries in new journals begins with the number following the last number in the completed journals. The numbering of entries in the journals for each name of the precursor is carried out within a calendar year in ascending order of numbers. The numbering of entries in new journals begins with the number following the last number in the filled journals. Journal pages not used in the current calendar year are crossed out and not used in the next calendar year. Unused magazine pages in the current calendar year are crossed out and not used in the next calendar year. The entry in the logs of each performed operation is certified by the signature of the person responsible for their maintenance and storage, indicating the surname and initials. The entry in the logs of each performed operation is certified by the signature of the person responsible for their maintenance and storage, indicating the surname and initials.

The procedure for maintaining and storing the journal Corrections in the journals are certified by the signature of the person responsible for their maintenance and storage. Erasures and uncertified corrections in the logs are not allowed. Corrections in the logs are certified by the signature of the person responsible for their maintenance and storage. Erasures and uncertified corrections in the logs are not permitted. The journal is stored in a metal cabinet (safe), the keys to which are held by the person responsible for maintaining and storing the journal. The journal is stored in a metal cabinet (safe), the keys to which are held by the person responsible for maintaining and storing the journal. Completed journals, along with documents confirming the implementation of transactions, are stored by a legal entity or individual entrepreneur for 10 years after the last entry was made in them. Completed journals, along with documents confirming the implementation of transactions, are stored by a legal entity or individual entrepreneur for 10 years after entering into them the last entry. After the expiration of the specified period, the magazines are subject to destruction according to the act approved by the head of the legal entity. After the specified period, the magazines are subject to destruction according to the act approved by the head of the legal entity.

The register of transactions related to the circulation of drugs for medical use is maintained by 1) drug manufacturers and drug wholesalers 2) pharmacy organizations and individual entrepreneurs licensed to pharmaceutical activities; 3) medical organizations and individual entrepreneurs licensed to practice medicine

The procedure for maintaining journals Registration of transactions related to the circulation of drugs is carried out for each trade name of a drug (for each individual dosage and dosage form) on a separate expanded sheet of the accounting journal or in a separate accounting journal on paper or in in electronic format Registration of transactions related to the circulation of drugs is carried out by persons authorized by the head of a legal entity to maintain and store accounting logs, or by an individual entrepreneur who has a license for pharmaceutical activity or medical activity.

The procedure for maintaining journals Accounting journals, filled in on paper, are stitched, numbered and sealed with the signature of the head of the legal entity (individual entrepreneur) and the seal of the legal entity (individual entrepreneur) before starting to maintain them. Accounting journals are issued for a calendar year. Sheets of accounting journals, filled in in electronic form, are printed monthly, numbered, signed by a person authorized to maintain and store accounting journals, and are stitched by the names of drugs, dosage, dosage form. At the end of the calendar year, the bound sheets are drawn up in a journal, sealed with an indication of the number of sheets and certified by the signature of the person authorized to maintain and store accounting journals, the head of the legal entity (individual entrepreneur) and the seal of the legal entity (individual entrepreneur).

The procedure for maintaining journals Entries in the accounting journals are made by a person authorized to maintain and store the accounting journal, with a ballpoint pen (ink) at the end of the working day on the basis of documents confirming the performance of incoming and outgoing transactions with drugs. The receipt of drugs is reflected in the accounting log for each receipt document separately, indicating the number and date. Drug consumption is recorded daily. Pharmacy organizations and individual entrepreneurs licensed for pharmaceutical activities, record the daily consumption of drugs, indicating separately for prescriptions issued medical professionals, and according to the requirements of medical organizations.

Procedure for keeping logs Corrections in accounting logs are certified by the signature of a person authorized to maintain and store accounting logs. Erasures and uncertified corrections in the accounting logs are not allowed. On the last day of each month, the person authorized to maintain and store accounting logs reconciles the actual availability of drugs with their balance in the accounting log and makes the appropriate entries in the accounting log. The accounting log is kept in a metal cabinet (safe), the keys to which are held by a person authorized to maintain and store the accounting log. Receipt and expense documents (their copies) are filed in the order of their receipt by date and are stored together with the accounting journal. The completed accounting logs are stored in the archive of a legal entity (individual entrepreneur).

Order of the Ministry of Health of the Russian Federation dated 284 "On the approval of the norms natural loss Medicines and medical devices in pharmacy organizations, regardless of the organizational and legal form and form of ownership "

Natural loss is the loss of goods due to natural processes causing a change in the quantity of goods (shrinkage, shrinkage, leakage, etc.) are commodity losses caused by natural processes that cause a change in the quantity of goods (shrinkage, shrinkage, leakage, etc.) Norms of natural loss are applied only in cases of shortage of the specified commodity and material values during the inventory The rates of natural loss are applied only in cases of shortage of the specified inventories during the inventory of drugs that have become unusable as a result of improper storage or careless handling, the rate of natural loss does not include drugs that have become unusable in as a result of improper storage or careless handling, the rate of natural loss is not included The specified standards do not apply to finished drugs industrial production... These norms do not apply to finished pharmaceutical products of industrial production.

Norms of natural loss for narcotic drugs, psychotropic substances and their precursors, drugs of lists of potent and toxic substances, other drugs subject to quantitative accounting, and ethyl alcohol are set for the amount consumed and depend on the type of consumption Norms of natural loss for narcotic drugs, psychotropic substances and their precursors, drugs, lists of potent and toxic substances, other drugs subject to quantitative accounting, and ethyl alcohol are set to the amount consumed and depend on the type of consumption

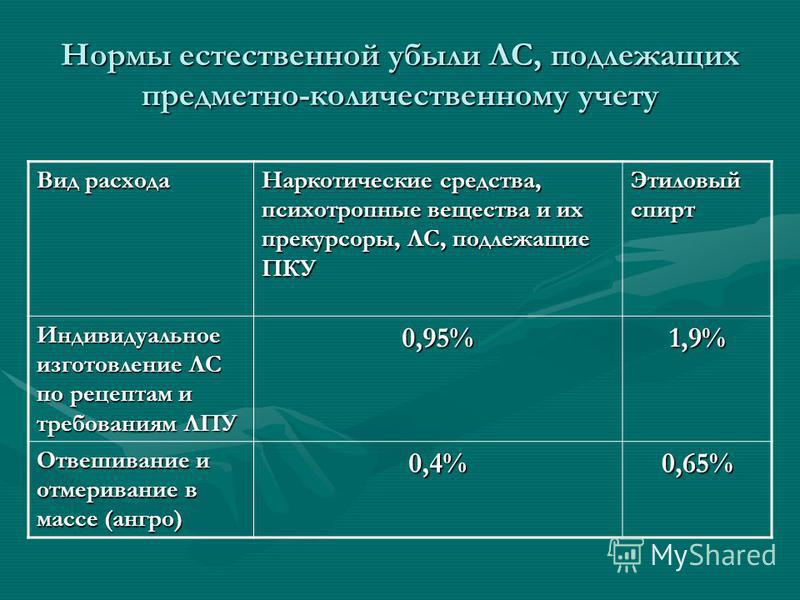
Norms of natural loss of drugs subject to quantitative accounting Type of consumption Narcotic drugs, psychotropic substances and their precursors, drugs subject to PKU Ethyl alcohol Individual production of drugs according to recipes and requirements of health care facilities 0.95% 1.9% Weighing and measuring in bulk (Angro ) 0.4% 0.65%



Features of the manufacture of medicines in pharmacies; a variety of prescriptions for drugs in composition and types of dosage forms; a variety of prescriptions for medicines in terms of composition and types of dosage forms; the complexity of the composition of manufactured drugs; the complexity of the composition of manufactured drugs; relative instability of manufactured drugs; relative instability of manufactured drugs; the need to combine the manufacture of drugs of different composition in one workplace; the need to combine the manufacture of drugs of different composition at one workplace; high costs and low profitability of drug manufacture High costs and low profitability of drug manufacture

Requirements for the organization of manufacturing special premises - assistant rooms special premises - assistant rooms special pharmacy furniture and devices special pharmacy furniture and devices safes (special cabinets) for storing poisonous, narcotic drugs and ethyl alcohol safes (special cabinets) for storing poisonous, narcotic drugs and ethyl alcohol measuring instruments for weight, volume and specific gravity means for measuring weight, volume and specific gravity Reagents for chemical control of drugs Reagents for chemical control of drugs

Workplaces for Pharmacists Manufacturing: Liquid Medicines for internal use; liquid medicines for internal use; powders and pills; powders and pills; ointments and liquid dosage forms for external use; ointments and liquid dosage forms for external use; medicines manufactured under aseptic conditions; medicines manufactured under aseptic conditions.

Rules for the admission of persons to work with narcotic drugs and psychotropic substances (Decree of the Government of the Russian Federation of the city of 892) The admission of pharmacy workers to work with narcotic drugs and psychotropic substances is carried out by the head pharmacy organization and provides for inclusion in employment contracts mutual obligations of the administration and persons associated with the circulation of narcotic drugs and psychotropic substances. The admission of pharmacy workers to work with narcotic drugs and psychotropic substances is carried out by the head of the pharmacy organization and provides for the inclusion in labor contracts of mutual obligations of the administration and persons associated with the circulation of narcotic drugs and psychotropic substances ... Persons under the age of 18 are not allowed to work with narcotic drugs and psychotropic substances. Persons under the age of 18 are not allowed to work with narcotic drugs and psychotropic substances.

Manufacturing of drugs containing narcotic drugs, poisonous and potent substances The issuance of narcotic drugs to the assistant room for the current work should be carried out only by a materially responsible person authorized to do so The issuance of narcotic drugs to the assistant room for current work should be carried out only by a materially responsible person authorized to do so. in the assistant room of pharmacies, stocks of narcotic drugs should not exceed daily requirement In the assistant room of pharmacies, stocks of narcotic drugs should not exceed the daily requirement. Pharmacist receives narcotic and poisonous drugs from the head of the department or his deputy. Pharmacist receives narcotic and poisonous drugs from the head of the department or his deputy. Medicines and pharmacist's signature On the reverse side of the prescription, the name and quantity of the weighed medicinal product are noted, the signature of the dispensed medicinal product and the pharmacist's signature are affixed The suspended substance is immediately used for the preparation of the medicinal product, which, after preparation, is immediately transferred to control and stored until dispensing in a special lockable cabinet. for the manufacture of DF, which, after preparation, is immediately transferred to control and stored until dispensing in a special lockable cabinet. At the end of the working day, narcotic and psychotropic drugs must be returned to the place of the main storage of narcotic and psychotropic drugs. At the end of the working day, narcotic and psychotropic drugs must be returned to the place of the main storage of narcotic and psychotropic drugs.

The shelf life and storage conditions of medicines manufactured in pharmacies are approved by order of the Ministry of Health of the Russian Federation dated 214

Organization of aseptic preparation of medicines solutions for injections and infusions, eye drops and ointments, medicines for newborns, individual solutions for external use, etc. For the production of medicines under aseptic conditions, a pharmacy must be equipped with an aseptic block solutions for injections and infusions, eye drops and ointments , medicines for newborns, individual solutions for external use, etc. For the manufacture of medicines in aseptic conditions, the pharmacy must be equipped with an aseptic unit (pr. Ministry of Health of the Russian Federation from see the textbook)

Sanitary Requirements for Manufacturing Medicines under Aseptic Conditions Order of the Ministry of Health of the Russian Federation dated No. 309 established sanitary requirements for manufacturing medicines under aseptic conditions. (see the textbook) Order of the Ministry of Health of the Russian Federation from No. 309 established sanitary requirements for the manufacture of medicines under aseptic conditions. (see tutorial)

Rules for the training and behavior of personnel in the aseptic unit Order of the Ministry of Health of the Russian Federation dated 309 also approved the rules for preparing personnel for work and rules of conduct in the aseptic unit (see the textbook). The Order of the Ministry of Health of the Russian Federation dated 309 also approved the rules for training personnel for work and rules of conduct. in the aseptic unit (see textbook)

Sanitary requirements for the manufacture of non-sterile dosage forms Order of the Ministry of Health of the Russian Federation from the city of 309 See the textbook Order of the Ministry of Health of the Russian Federation from the city of 309 See the textbook

Organization of production in pharmacies of concentrates, semi-finished products and intra-pharmaceutical preparations Concentrated solutions (concentrates) are pre-prepared solutions medicinal substances more high concentration than the concentration in which these substances are prescribed in prescriptions. Concentrated solutions (concentrates) are pre-prepared solutions of medicinal substances of a higher concentration than the concentration in which these substances are prescribed in prescriptions.

Organization of production in pharmacies of concentrates, semi-finished products and intra-pharmacy billets Semi-finished products are an overdosed type of billet used in a mixture with other ingredients, which is part of Complex dosage form Semi-finished products are an under-dosed type of workpiece used in a mixture with other ingredients, which is an integral part of a complex dosage form.

Organization of manufacture in pharmacies of concentrates, semi-finished products and intra-pharmacy preparation Intra-pharmaceutical preparation is the preliminary preparation of dosage forms according to frequently occurring prescription prescriptions. Intra-pharmaceutical preparation is the preliminary production of dosage forms according to frequently encountered prescription prescriptions. Intra-pharmacy packaging - dispensing drugs in quantities suitable for dispensing to customers. Intra-pharmacy packaging - dispensing drugs in quantities suitable for dispensing to customers.

The manufacture of concentrates, semi-finished products and intra-pharmacy blanks in a pharmacy is called laboratory, and intra-pharmacy packaging is called filling work. Laboratory and filling work is recorded in a special "Journal of laboratory and filling work" in an approved form. Laboratory and filling work is recorded in a special "Journal of laboratory and filling work. "According to the approved form

"Log book for laboratory and packaging work" The journal must be numbered, laced and sealed with the signature of the head of the pharmacy organization. The journal must be numbered, laced and sealed with the signature of the head of the pharmacy organization. and packing work is carried out separately in two journals The journal also takes into account the cost and amount of pure ethyl alcohol dispensed to the population by prescriptions The journal also takes into account the cost and quantity of pure ethyl alcohol dispensed to the population by prescriptions All records of medicines issued for the manufacture are made immediately after completion works and are sealed by the signatures of the persons who produced and accepted the work. All records of the medicines issued for the manufacture are made immediately after the completion of the work and are sealed by the signatures of the persons who produced and accepted the work


